Medical network - on January 4, drug research and development since 2015, a series of adjustment, in 2016, have already produced results: declare drug approval number decreased significantly, declare a backlog situation gradually ease. At the same time, although the product approval rate increased, but because of before a large number of withdrawal, makes the number of newly approved drugs also decreased obviously.
According to the state food and drug supervision and administration bureau (CFDA) 1 ~ 2016 November monthly approved drug announcement, as of November 2016, the CFDA approved drugs a total of only 192 (according to the approval Numbers, hereinafter the same), and all of 2014 and 2015, the year newly approved drugs are more than 300. According to the current progress of examination and approval, approval of new drugs in 2016 number will only be about two-thirds of the number two years before.
Medicine is still a drug research and development of the main categories, 2016 1 ~ 11 month approved 192 varieties, there are 175 belongs to the product. Number of biological medicine basic stable, approved a total of 12 varieties, but Chinese medicine approved by the decline in the number, only five varieties approved.
In the country is committed to strengthening against the background of traditional Chinese medicine, Chinese medicine research and development of lonely regret letting a person, what is the enterprise attaches great importance to the problem, policy of the executable, or the product itself? These need to traditional Chinese medicine thinking.
Than in previous years, low level repeated declaration has been eased significantly, more valuable and distinctive varieties approved, will bring more benefit to clinical.
New approved 2016 varieties of future value
For 192 varieties approved in 2016, based on the value of inventory of the most sought after by the market, looking forward to the patients, the most innovative value, most parents expect, most can enjoy two child policy dividend, the most traditional and most embarrassing seven categories of most varieties.
1, the most sought after by the market
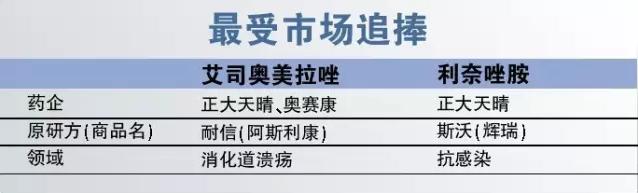
Escitalopram omeprazole (i.e., esso beauty pull azole) is digest the most popular in the field of medicine, as a proton pump inhibitor (PPI), the drug is widely used in many kinds of digestive tract ulcer and hemorrhagic diseases. Astrazeneca's original medicine nexium in 2015 samples of hospital sales reached 800 million yuan. And before 2016, in addition to the original drugs, only chongqing oral generic lai mei shu lai beauty. In 2016, zhengda shine and orsay kang vial escitalopram omeprazole in the same period. Considering the vial PPI market is bigger, so the two varieties is undoubtedly the market demand. Especially the orsay kang, as a domestic leading enterprise of PPI injection, escitalopram omeprazole injection of approved will bring huge sales promotion for it.
Rina thiazole amine belong to synthetic oxazole alkane ketone of antibiotics. Although belong to antibiotics, but even in the context of impedance, rina thiazole amine serious resistant infections is still great opportunity. 2015 samples of the original drugs Pfizer ", "hospital sales of nearly 70 million yuan, the first in imitation of a medicine, rina thiazole amine was listed at the end of 2015. Considering the serious drug-resistant infection were numerous, rina thiazole amine and huge market space, 2016 board approved time imitation medicine is also worth looking forward to the weather is fine.
2, looking forward to the patients
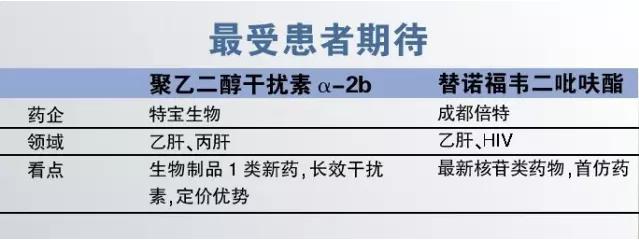
Although domestic for treatment guidelines are clear, such as hepatitis b and c will polyethylene glycol interferon alpha as first-line drugs, but in China for a long time peg-intron monopolized by the original drug, roche's pegasys and MSD palin to high prices, some patients can only choose a short-acting interferon injections for a long time. Fortunately, china-made long-acting interferon alpha finally approved 2016, pag bing (polyethylene glycol interferon alpha 2 b) is the treasure biological research and development of national class 1 new drug, biological products with completely independent intellectual property rights. On pricing, union than with the long-term alpha 2 b pelletier can lower 20% ~ 30%, can to a certain extent, reduce the burden of patients.
With star drug tenofovir for liver disease and liver disease patients with focus on products, such as drug resistance and turn negative aspects, tenofovir is by far the most excellent nucleoside drugs. Although tenofovir into China for many years, but early indications only HIV on one hand, on the other hand high price treatment costs more than 30 yuan (days) that makes the market cannot expand for a long time. In view of this, the patient can expect domestic tenofovir listed as soon as possible. Under this background, the chengdu times snatch the first successful imitation drugs, its price compared with the original drug is expected to decline significantly. "At the same time, the original drug viread" also slashed prices more than 67% in the national drug negotiations, some provinces and cities have begun to implement new prices, two positive factors will undoubtedly bring more hepatitis b patients choose tenofovir.
3, the most innovative value of domestic new drugs
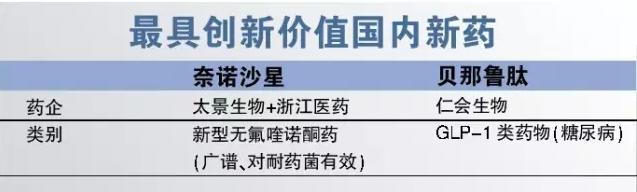
New molecular medicine class research and development is very difficult, but in 2016 the domestic research and development of new molecular drug, nai, the effect of success. Zhejiang medicine and Taiwan's view of joint development of nai, the effect of capsule (incumbent) is a new type of quinolone drugs, the drug antibacterial spectrum broad and effective for drug-resistant bacteria, not containing fluorine is safer. Considering the marketing side of zhejiang medicine and the original square too scene creatures are Chinese companies, so this is also the domestic independent research and development of new drugs listed a model of success. But for the future, nai, the effect of need more evidence-based research support.
Glp-1 drugs is a hot spot in the global diabetes drugs in recent years, the six successful products before have been granted, one of the most popular lalu peptide with annual sales of nearly $3 billion. At the end of 2016 the kernel will be independent research and development of biological bei CFDA approval of the peptide lu, become the first domestic listed on independent research and development of glp-1 drugs. Compared with the traditional oral drugs, glp-1 drugs hypoglycemic effect is strong, low risk of hypoglycemia, also can reduce weight, but the high cost of treatment to restrict its use at home, we are looking forward to that, lu peptide can greatly reduce the cost of treatment of glp-1 drugs, let more patients benefit.
4, most parents are looking forward to

Vaccine is an important way to prevent childhood pneumonia, and at present in the world's most popular vaccine is drug repeatedly for infantile pneumonia (pneumococcal polysaccharide conjugate vaccine). 7 pneumococcal conjugate vaccine (pei son 7) has been in the domestic market for many years, but 7 valence vaccine for more than 90 kinds of serotype pneumococcal resistance slightly insufficient, so the original Fang Huirui developed the upgrade version of the price of 13 vaccine pei son 13. However, during the reporting new pei son 13 at the same time, pei son 7 gradually stop supply, make often have parents online, offline for help pei channel. After years of examination and approval, pei son 13 finally won the approval of China, so the problems a lot of parents pneumonia vaccination problem will be solved.
5, the most enjoy two child policy dividends

Under the background of open up 2 children, increase the chance of pregnancy by drug intervention demand increased dramatically. For infertility as commonly used in the field of follicle stimulating hormone, market opportunity is further expanded. Follicle-stimulating hormone is divided into from urine urine follicle-stimulating hormone and recombinant follicle stimulating hormone, samples of the current domestic hospital follicle-stimulating hormone has sold more than 500 million yuan, but approved only five varieties, under the background of comprehensive and having two children, a new approved follicle-stimulating hormone will have larger market opportunities.
In 2016, Shanghai day wei biological urine follicle-stimulating hormone, it is the third approved domestic urine follicle-stimulating hormone, is expected to share two child policy brings market dividends.
6, the most traditional
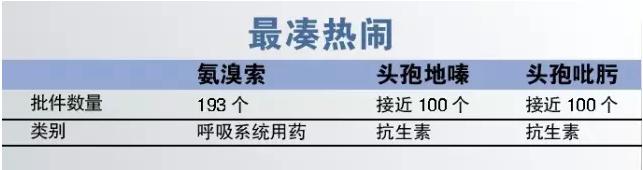
Cluster phenomenon has eased significantly in 2016, to declare for has been approved by the manufacturer to many varieties, new declaration is relatively rare. However, ammonia bromine, cephalosporins lamictal and cefepime etc. There are many new application approved.
Vial cephalosporins lamictal and approval documents with cefepime injections currently have nearly 100, Harbin pharmaceutical and suzhou 2 how to make the two varieties of new drug approvals from so many entrants in first chance to still need to think more.
Ammonia bromine is more serious in terms of repeated declaration, approval documents approved according to data from the CFDA ammonia bromine line has reached 193, including 2016 contributed 5, newly approved manufacturer can only demonstrated from have severely saturated market looking for opportunities.
7, the most embarrassing

Human papilloma vaccine, which is commonly known as the HPV vaccine, is currently the world's best-selling adult with vaccinations, research is generally believed that the vaccination will significantly reduce the incidence of cervical cancer and HPV related malignant tumor. However, the mainland HPV vaccine cannot be approved for a long time, it also cause the crowd had to choose to Hong Kong or China, Japan and South Korea.
In 2016, GSK bivalent human papilloma virus adsorption was finally approved in our country. But awkward, at exactly the same period, the price of 2 HPV closure of its sales in the United States. Similar to pneumonia vaccine, HPV also includes various subtypes, vaccine has been listed in the 2 price only effective for HPV16 and 18, resulting in immune is not completely, the late listed immune range greater price of 4 and 9 price to expand the scope of immune vaccine, vaccine is more reliable than 2 price. Although the price of 2 vaccine still can greatly reduce the risk of infection, but the delisting will surely make quite a few people ready for injection have doubt on the efficacy and safety.
2017 is expected to be listed's looking forward to breed
Along with the implementation of the priority review system, the difference of the drug approval rate has become increasingly apparent, more to encourage those who have higher level of innovation and clinical value of new drug development, especially attention, rare diseases and aging of knotty specific populations such as children of specific diseases. In addition, for the first drug, review progress will be accelerated, heralding in 2017 will have more desirable varieties approved.
For 2017 worth looking forward to the variety, we will be divided into the foreign drug innovation, independent innovation and high levels of generic drugs three categories.
1, the innovation medicine abroad
In 2017, is expected to domestic approved foreign innovation medicine, which includes the following four varieties of concern.
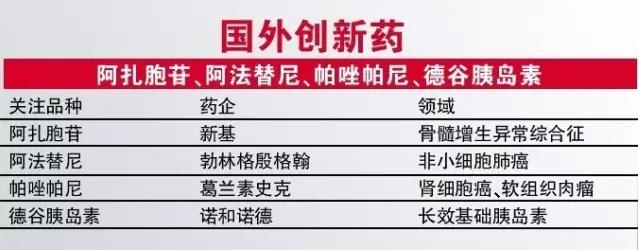
New base azacitidine is the world's first approved drugs for myelodysplastic syndrome (MDS), the varieties on the market in China will further enhance new product advantages in the field of blood, the lancet, a study is also prove the existence of azacitidine and drug lenalidomide combination of foundation.
Boehringer ingelheim TKI drugs of afar more target kinase inhibitor, on behalf of, is its effect on HER2 at the same time, also irrevocably inhibiting EGFR, the study found that the drug compared with Yi Rui sand and so on, can significantly improve the treatment effect of non-small cell lung cancer, so the drug obtained FDA breakthrough. Considering China's many lung cancer group, the method for he has a huge market opportunity.
Palmer azole panitan bake azole (mpa) was developed by GSK for renal cell carcinoma and soft tissue sarcomas TKI drugs, the New England journal of a study that palmer's treatment of renal cell carcinoma and chougny for similar, but better security, palmer thiazole panitan also believed to be better treatment of soft tissue sarcomas.
Compared with the former three cancer drugs, the author pay more attention to DE valley, the research progress of insulin. DE valley is following the insulin and insulin, insulin insulin after another long-term basis. Compared with insulin, insulin DE valley has a lower risk of hypoglycemia, and convenient for clinical use (DE valley insulin dosing interval 8 to 40 hours does not affect the validity and security), the original party hopes to use its challenge is dominant.
2, the domestic innovation
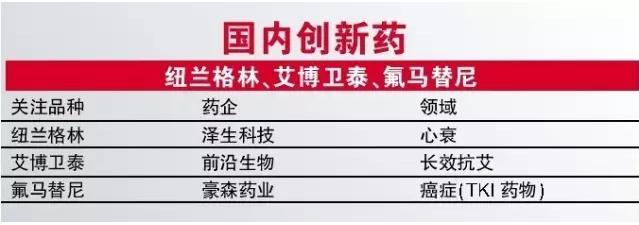
For newland and YiBoWei Thai green, seems to be from the listed are only poor finishing, but seems to have is far worse. Both first - in - class breed already completed clinical research, production. Newland belongs to green heart failure drugs, its mechanism and existing drugs are not the same, and YiBoWei ty is considered is expected to become the first effective AIDS drugs. From the point of innovation and clinical value, the two drugs are extremely valuable, once approved, will undoubtedly become the representative of the domestic new drug innovation, but precisely because two varieties belong to the first - in - class, so the two species would be approved, we could just expressed cautious optimism.
TKI is a hotspot of domestic innovation medicine, while for ek and path for listed success also prove that the domestic research and development of TKI drugs. Fluorine of horses for his declaration production stage at present, the drug on the target is the Bcr - Abl, can be used for Glenn WeiNai medicine group of patients, a fluorine horse for and imatinib in the treatment of CML, randomized controlled studies have shown that fluorine horse for his effect is better.
3, the high level of generic drugs
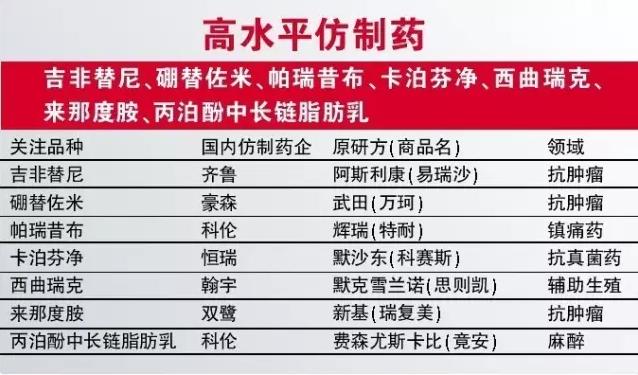
In addition to the innovative medicine, some is expected to be approved in 2017 generic drugs we also pay close attention to. According to priority review directory, we prefer a few blockbuster drugs on the list first imitation drugs, such as the treatment of qilu, boron for zc of meters, koren paranal yesterday cloth and hengrui mooring Finn net card. Other desirable varieties include shenzhen squire first assisted reproduction medicine west QuRuiKe, double heron's closely watched the first copy the drug lenalidomide and koren anaesthetic propofol/long-chain fat emulsion in the new dosage form.
|
