Medical network - on January 4, medical apparatus and instruments in recent years has received the attention from all walks of life, compared with 2014, when China's medical equipment industry in 2015 fell slightly a little bit, but the growth rate is still the world's average speed of three times, and the layout of the medical equipment is an important part of "much starker choices-and graver consequences-in planning, medical equipment industry is facing a good opportunity for a lifetime.
According to the regulations on the supervision and administration of medical devices by the state council provisions of article 4 the state shall implement a medical apparatus and instruments according to the degree of risk classification management, the third kind is with high risk, the need to take special measures to strictly control the management to ensure the safe and effective medical devices.
In general, Ⅲ implanted medical devices "refers to the human body, to support and sustain life, or potentially dangerous to the human body, its security, effectiveness must be strictly control the medical apparatus and instruments, such as cardiovascular interventional devices, we are familiar with nuclear magnetic resonance (NMR) MRI and bone implant material, etc.
According to the current data to infer that, compared with the previous two years, the number of China's medical device registration approval in 2016 will still maintain a certain decline, but overall growth is still far exceeds the overall growth of the world.
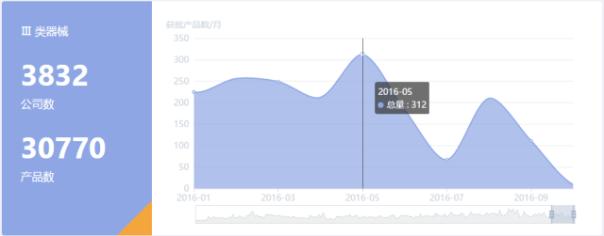
Ⅲ class of 2016, China's medical equipment (including in vitro diagnostic reagents) approved of as shown, which maintained a high approval rate during the first half, reached the highest number of approved in May, compared in the second half in the first half fell.
Among them, the approval number of the top five were implanted materials and artificial organs, medical polymer materials and products, medical optical instruments, instruments and endoscope equipment, interventional equipment and extracorporeal circulation and blood processing equipment.
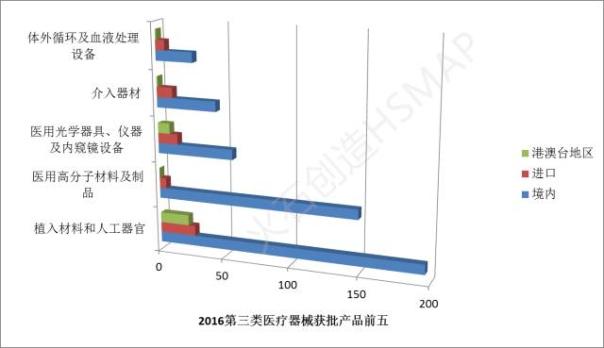
Compared with the examination and approval of the 2015 found that the implanted materials and artificial organs, medical polymer materials and products, medical optical instruments, instruments and endoscope equipment, intervention equipment categories continue to occupy a larger proportion.
At the same time, due to the country during the period of "much starker choices-and graver consequences-in" pay more attention to the development of medical equipment industry and strong support, especially the tissue repair and regeneration materials, artificial organs and life support equipment, etc., are considered development key, so in the transition from "twelfth five-year" to "much starker choices-and graver consequences-in" in 2016, implant materials and artificial organs approved more than medical polymer materials and products, has become the most approved of the category.
Officially issued by the state council on December 19, "" much starker choices-and graver consequences-in" national strategic emerging industry development planning ", which was mentioned to "using the material manufacture and so on new technology, accelerate the tissue repair and replacement material and interventional medical device product innovation and industrialization." Which further proves the author's reasoning. By 2018, our country orthopaedic implant materials market scale will reach 21.2 billion yuan.
Let's see, in the top three categories, each category of medical devices and how is approved.
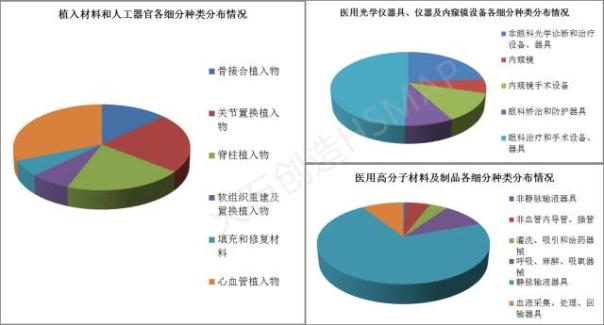
Recently released "" much starker choices-and graver consequences-in" science and technology innovation plan "put forward, in the direction of biomedical materials, to tissue replacement and repair function, the intelligent control for the direction, speed up the 3 d printing, material surface biological functionalization and modification, a new generation of biological material inspection evaluation method and key technological breakthroughs, such as key layout can be induced biomedical materials, tissue engineering products, a new generation of interventional medical apparatus and instruments, artificial organs and other major strategic products. The current applications and research hot spot and focus in orthopedic bone implants and implant devices, especially popular 3 d printing, the current domestic has approved the registration of 3 d printing implantable orthopaedic medical apparatus and instruments, believe that as technology constantly breakthroughs and mature, combined with national policy support and incentives, implanted materials and artificial organs the categories of medical equipment industry will have great development.
In high-end medical imaging products data so far, the author found that 2016 approved several X-ray computer tomography (CT), ultrasound diagnostic equipment, medical magnetic resonance imaging (MRI) equipment, radioactive nuclide diagnostic equipment and laser equipment are all domestic equipment.
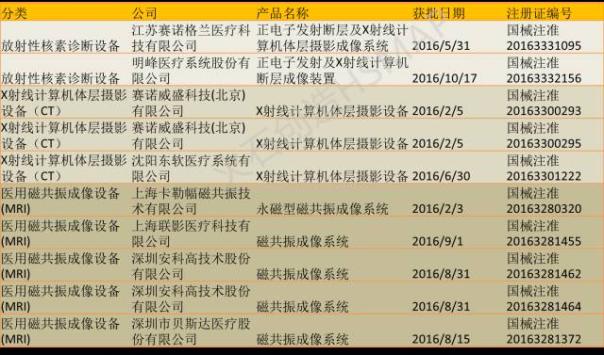
In 2016 approved by the part of the medical imaging products
Source: HSMAP system
For a long time, in the high performance medical device, China market almost monopoly by imported products, after years of efforts, our country in these areas have also made many breakthroughs. "Twelfth five-year" period of trying to make a lot of product for import substitution, but on the core components, we still need to spend a lot of energy to achieve breakthrough, so "much starker choices-and graver consequences-in" the primary goal of the medical equipment planning is reality instead of imported product, with localization, high-end, branding, international into the direction, promote leapfrog development.
After years of development, China's medical equipment market has begun to take shape, pharmaceutical and industrial enterprises above designated size in the first half of 2016, medical equipment and instruments this sector is the second fast profit growth, after chemical active pharmaceutical ingredients. At present, the national total of 37 medical instrument company listed on the Shanghai stock exchange and shenzhen stock exchange, the business scope contains Ⅲ class equipment (including in vitro diagnostic reagents) with a total of 23, third quarter net profit is as shown:
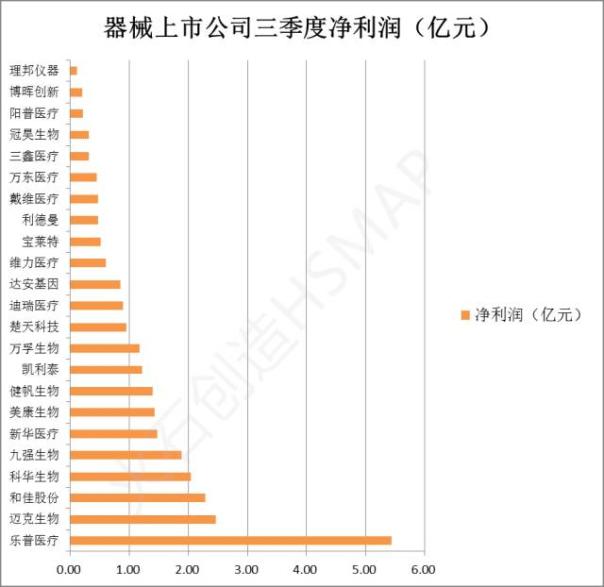
Source: Oriental wealth
Global pharmaceutical and medical equipment, the proportion of consumption for the 1-0. 7, by comparison of China the figure was only 1-0. 19, coupled with global consumption proportion also there are still expanding trend, China's medical equipment market will be very broad development space. In a series of policies to accelerate the country as well as the growing market demand, driven by China's overall layout of the medical devices convert from low-end to high-end products with high added value.
Data sources:
The state council "2015 annual report on the work of the medical device registration"
Flint create HSMAP system
The CFDA website
Eastern net wealth |
