Medical network - January 5, on January 4, the CFDA release on the drug clinical trial data check list check the announcement of registration of said, decided to new received 14 completed clinical trials to declare manufactured or imported drug registration application for clinical trial data for verification.
Announcement, points out that before the administration of state food and drug supervision and administration organization verification, inspection found that the drug registration applicant drug clinical trial data authenticity problems, should take the initiative to withdraw the application for registration, the state food and drug supervision and administration bureau announced its list, shall be investigated for their responsibility.
Administration of state food and drug supervision and administration of the food and drug audit inspection center will be published on its website that the inspection plan, and inform the applicant for drug registration and the local provincial food and drug regulatory authority, the public 10 working days after the centre will inform the inspection date, no longer accept drug registration the applicant's application for withdrawal.
Administration of state food and drug supervision and administration of drug clinical trial data will be found in the inspection data of the applicant for pharmaceutical clinical test shall be given heavier processing partners and managers, contract research organization partners, and failed to effectively takes office will be the responsibility of the food and drug regulatory inspectors.
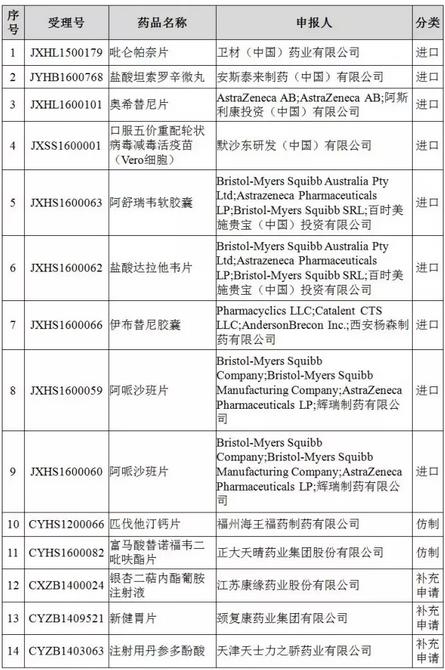
14 drug clinical trial data check list for inspection registration list
|
