Medical network - on January 13, 2017, is still the drug released annual manufacturing research and development production policy.
On January 10, the CFDA drug approval center released about the listed chemicals production process change research technical guiding principles for advice notice (hereinafter referred to as the "2017 guidelines"). This is since 2008 the former state food and drug administration issued the listed technology guiding principle of the study on chemical changes (a) "(hereinafter referred to as the" 2008 guidelines ") for the first time since the adjustment.
"Guidelines", 2017, the main production process according to raw material production and preparation production Angle change research, and the key to active pharmaceutical ingredients and injection, oral solid preparation of three kinds of chemical drugs on the market mainstream formulations formulation research and information requirements change. As "guidelines", 2017, August 2016 CFDA launched by the pharmaceutical production process check work will be carried out in the above three dosage forms is preferred.
Two big bright spot
First, with the international current standards
"2017 guidelines" very pay attention to with the international current standards, in three aspects:
First, after the generic drug production process change, emphasis on equality or equivalence with the original product, evaluation methods must be in accordance with the current standards.
Second, multiple reference internationally accepted guidelines, such as new API impurity control reference ICH quality limit prescribed by the Q3. References also covers the FDA and EMEA, TGA and ICH guidelines.
Third, pay attention to the pharmacopoeia standard contains products at home and abroad, and asked for the original approved quality standards, the current edition of China pharmacopoeia standards and comparing the current mainstream version of foreign pharmacopoeia standard.
In depth
"2017 guidelines" requirements for the quality of production process changed the original product is consistent, and assessment method with the international current standards, this with the national implementation of generic drugs at present consistent with the direction of policy evaluation. With the technological change process check work, study will be reversed transmission of oral solid preparations and injections are consistent with the original product quality research, clinical equivalence.
According to the process of check schedule, pharmaceutical production enterprises shall be completed before June 30, 2017 in product production technology research and validation, submit supplementary application and so on related work, no other products should be completed before December 31, 2017 the work; Did not finish, should stop production.
"2017 guidelines" increased in line with the original request, is expected to have some products production. Not in the first 2018 consistency evaluation must be completed in 2018 catalog of products should be actively started consistency evaluation.
Second, pay more attention to process control
1, key reference 2008 version of the three projects
"2008 guidelines" process change research can be divided into active pharmaceutical ingredients in the production process changes, drug formulation for requirement of medicinal materials and preparation process change, change, specification change registration standard, valid, and storage conditions change, drug packaging materials and containers of change, the imported drugs the change of origin and import API origin and imported drugs used API changes, changes of domestic drug production preparation of origin API origin ten projects.
Released in August 2016 the implementation of pharmaceutical production process of checking the job announcement, the Ⅰ class change, Ⅱ changes and Ⅲ change is divided more reference "guidelines" 2008 "change API production process" and "drug preparation preparation production process", basic didn't mention the rest of the project.
"Guidelines", 2017, in addition to refer to change the production technology of active pharmaceutical ingredients and preparations change "drug production process", also refer to the "2008 guidelines" in the "change prescription drug preparations for the requirements of the medicinal materials", and integrate in the "change medicine preparation production process".
2, increasing the process key point
"2008 guidelines" is in the top ten projects under class Ⅰ changes, Ⅱ changes and Ⅲ change level corresponding process change details.
"2017 guidelines" is in the change of API production process and change medicine preparation production process under the two projects increase the division of key points of process change process, and then the key points on the tiny segment changes (i.e. Ⅰ class changes), medium change (i.e. Ⅱ class changes), and significant changes (i.e. Ⅲ class changes) corresponding to the different levels of detail.
Among them, the change of API production process including changes to the production line (such as shorten, extend or adjust the production line, change the starting reagents and raw materials, etc.), change production conditions (such as the change in mixing method and the way of drying process principle, inventory, reaction temperature, reaction time, stirring speed, stirring process parameters such as time changes, change material control/process control (such as change agents, source of starting materials, preparation technology, quality control, etc.) and other possible changes.
Chemical production process changes mainly include change accessories (source, type, level, dosage, species, etc.), change, change production equipment preparation (process principle changes such as dry granule and the interconversion of the wet legal system grain, process condition change such as drying temperature, tablet hardness, etc.), change preparation production process quality control methods and their limits (intermediates quality standards change, the change of the process inspection), etc.
To increase the key points of process change process, let the "guidelines", 2017 more close to the thinking of pharmaceutical engineering.
3, three levels to categorize the content more specific details
Increase "key point" of process change process after this one dimension, "2017 guidelines" combined with small changes, moderate changes and significant changes of three level of analyzing the influence of different details, more embodies different process change the details of the content, the influence of the process change can cause what kind of level so as to study the effect of the validation.
For example in the "guidelines", 2008, materials consumption change is on Ⅱ class changes, and in the "guidelines", 2017, excipients dosage change but there are three levels of change, and correlated with degree of the three levels of change and dosage form (see table 1, 2, 3).
Table 1 Ⅰ class changes (i.e., minor changes)
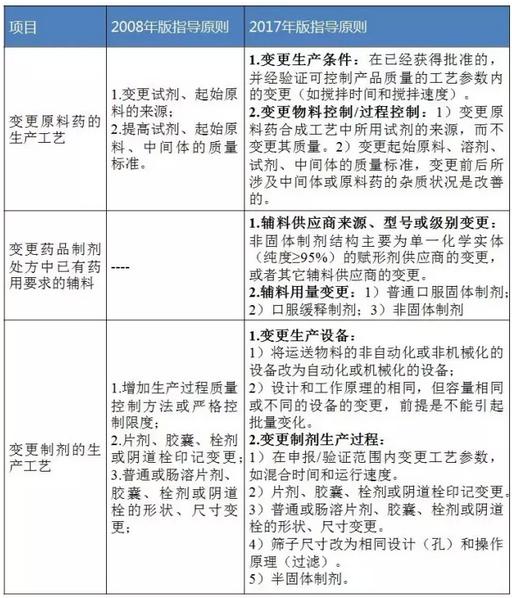
Data source: identify sensitive information (note: "carry out the work of drug production process to check about the announcement" relevant content reference "guiding principles" in 2008, except accessories. "2017 guidelines" will be complementary makings change content on "change medicine preparation process section. The same below.)
Table 2 Ⅱ class changes (i.e., moderate changes)
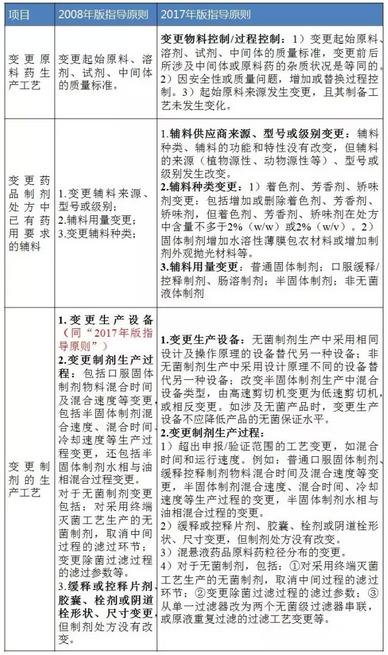
Data sources: identify sensitive information
Table 3 Ⅲ class changes (i.e., significant changes)
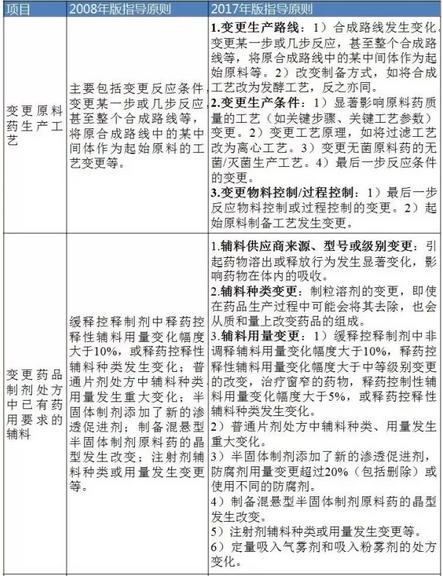

Data sources: identify sensitive information
Materials dosage change of tiny change: refers to the don't have any effect on product quality and performance. Changes before and after drug dissolution/release behavior consistent, or in connection with the body absorb and curative effect of important physical and chemical properties and indicators are consistent. In addition to the product appearance, change after drug quality standard not changed or more strictly.
Materials dosage change of medium change: refers to the changes before and after drug dissolution/release behavior consistent, or in connection with the body to absorb and curative effect of important physical and chemical properties and indicators are consistent. In addition to the product appearance, change after drug quality standard not changed or more strictly.
Materials dosage change of major change: refers to drug quality may have a significant impact. Such as half solid preparation has added new penetration promoter; Semi-solid preparations preservatives usage changes more than 20% (including deleted), or use different preservatives. Preparation of suspension type of half solid preparation API crystal type change.
In depth
"2017 guidelines" in a more comprehensive to study from the point of view of process design and process management to verify production process change on the influence of drug safety, efficacy and quality controllable. Enterprises in learning "2017 guidelines", should be at the same time grasp the CFDA management from fragmentation to the process systematic change.
future
1. Is expected to carry out the work of pharmaceutical production process to check on the announcement of traditional Chinese medicines and biological products mentioned guidelines will be released.
2. "2017 guidelines" rigorous degrees from the 2008 version, embodies the national promotion generic drug quality determination, for chemical medicine production enterprises set up a new threshold. Check the flight inspection process will be exposed some irregularities in the production of enterprises, technology check will become new reshuffle big production enterprises.
3. The chemical medicine enterprises should actively improve competitiveness and to the international standards, for in the fittest competition "with the king." |
