Medical network - Jan. 13 January 11, state food drug safety administration office to release the public general name for proprietary Chinese medicine technology guidance of principles (draft) ", requests the naming of new drug of TCM, as well as the original proprietary Chinese medicine is not standard, must conform to the guiding principle -- that is, there are a number of proprietary Chinese medicine or will face mandatory change name, it is no small matter for proprietary Chinese medicine enterprise!
▍ 5000 proprietary Chinese medicine or face mandatory change name!
Draft released four naming principles, respectively is: 1, the principle of "science and concise, avoid the nuptial"; 2, "necessary and reasonable" principle; 3, "avoid hinted, exaggerated effect" principle; 4, the principle of "embody traditional culture characteristic".
Among them, the principle of "science and concise, avoid the nuptial" in one paragraph, should not adopt the feudal superstition or vulgar language. What word can be defined as "feudal superstition" or "vulgar indecent"? This is puzzling. For example, like a day die away, with a smile and a half step, this kind of work?
"Avoid hinted, exaggerated effect" principle, in the name to avoid containing "hypoglycemic, antihypertensive, lipid, diminish inflammation, cancer", etc. Should not be exaggerated, boast, unrealistic. Such as: "the treasure", "spirit", "pure" "strong" "available", etc.; The name contains "drive" and "secret", etc.
The more interesting, so why not write "sugar", "decompression", "air", "blood" can? Currently on the market contain the keywords of the drug is not a few. Our common spirit wind, rhinitis, puissant loquat... All these common household standing drugs, will be renamed?
, according to a dandelion CFDA drug database, containing "avoid hinted, exaggerated effect" principle of keywords of proprietary Chinese medicine article 5326 approval, not including the remaining three principles, does that mean the drug is likely to face the fate of the name.
After change the name, packaging, manuals, small boxes, cartons, labels, all want to change, going to the food and drug administration for the record, advertising also need to prepare audit data. Drug name for drug firms is a big project.
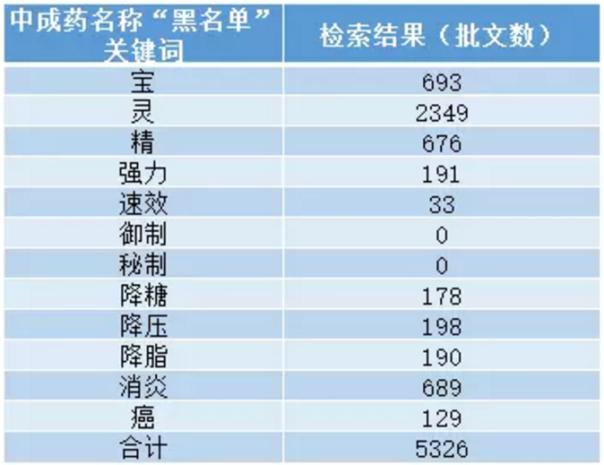
(source: dandelion statistics)
Before, there are a lot of pharmaceutical drug firms are willing to take the initiative to change the name, but the change of some drug companies will produce the same kind of drug dosage forms or specifications for a change, a new application for registration of a new brand name. The higher drug prices in "new" way, to obtain greater economic benefits. Some name a variety of drugs are actually the same kind of medicine, ordinary consumers if you don't know this, when they are ill often take the different names of the same kind of medicine, cause excessive doses, can pose a big threat to life.
Now, not you take the initiative to change, is a country requires you to change, and it is must be changed by the regulation, it is not the same. For some, such as "wind" the names of the pharmaceuticals such already thorough popular feeling, renamed maybe not a good thing. |
