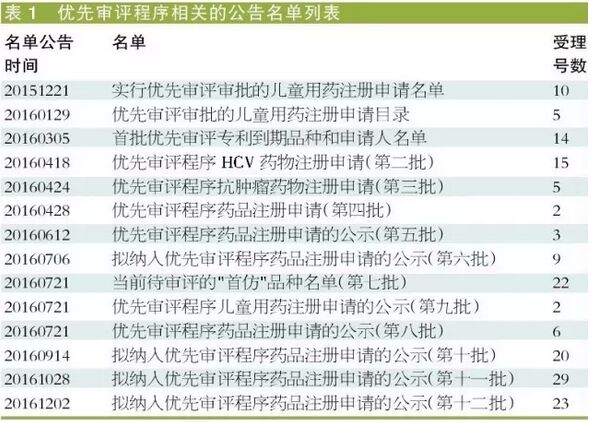
Medical network February 6 - since December 2015, CDE unveiled a priority review procedures related announcement list total of 14, a total of 165 accept order time of the public, involving 157 to accept the number. In CDE "information disclosure - priority review approval -" priority review varieties list "of the communist party of China, published 150 to accept order with" quasi "priority review procedure" consistent product name and applicant number 118 to accept the time, corresponding to 107 to accept the number. This means that after entering the priority review procedure will eventually into varieties of priority review list the success rate of nearly seventy percent, also means that the failure rate of nearly thirty percent.
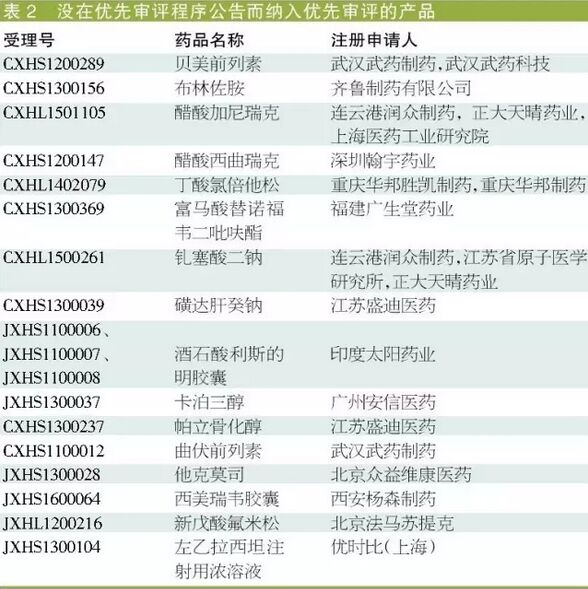
API has the varieties that are attached to the priority review. In 18 accept order without announcement in the priority review program of "priority review of varieties, 12 are active pharmaceutical ingredients. In addition to the card berth flumetasone three new amyl alcohol and acid, other API corresponding dosage form products are included in the priority review of varieties.
1 the public batch success rate
Salty reach data V3.2 found that, from the perspective of success, proposed "priority review of publication of the application for drug registration (11) list will eventually enter the lowest priority review success rate, 29 products only one was identified as a priority review. Implement priority review, published in 2015 for examination and approval of children for drug registration list of failure rate is relatively high, at 60%.
The success rate of one hundred percent: quasi "priority review of publication of the application for drug registration (10), success rate of 65%; Publication of the quasi "priority review of applications for drug registration (12), the success rate of 87%; Publication of the quasi "priority review of applications for drug registration (6), success rate of 89%; The current review of varieties of "imitation" list (7) the success rate of 91%.
2 "double quote", first copy will be rejected
Overseas listed generic synchronous application in domestic market, jiangsu XMC technology of fluorine of [18 f] deoxidization and glucose injection has not approved priority review; The European Union has approved, has passed the FDA certification of hainan company pharmaceutical, more yesterday galloway sodium for injection and synchronous to submit application for the registration, the company has through the eu GMP inspection for injection panxi tora azole sodium, didn't get priority review; The European Union has approved the hydrochloric acid of sichuan hui yu pharmaceutical made for kang injection has not approved priority review.
However, hainan split synchronous submit us ANDA application, has passed the FDA inspections of azithromycin for injection, sichuan hui yu has submitted an application for registration in the eu and the UK MHRA GMP certification for injection pemetrexed disodium, have been incorporated in the varieties of priority review list.
It seems that in the United States or the British public is more likely to get priority in the review.
"Priority review list of varieties and belong to the overseas listed synchronization application in domestic market of generics are: valsartan tablets of zhejiang huahai pharmaceutical co., and Shanghai AnBiSheng pharmaceutical meng LuSi sodium chewable tablet.
In addition, not all varieties of "imitation" can enter the priority review, such as the weight of jiangsu waterlooville pharmaceutical pharmaceutical tartaric acid rivastigmine, zhengda shine the rina thiazole amine injection, jiangsu hengrui medicine injection with paclitaxel (albumin combined type), the above products have a common - branded drugs has been listed in China.
3, hepatitis c drug failure rate are highest
Treatment areas, children's drugs and hepatitis c treatment medicine is priority review is most likely to die.
Children's drugs, the injection port b raschig, salmeterol assigned powder inhaler, pediatric MaLong products such as cough and asthma particles finally is not included in the priority review.
Hepatitis c treatment drug, Sarah rui wei potassium tablets, according to he wei capsule, phosphate phosphorus cloth vevey palmer he wei, TP - 168 and ZN2007Na pills didn't get priority review.
However, this was the first listed drugs "special review". Not listed in the domestic sales of extracted from plant, animal, mineral substances such as the effective component and its preparation, the medicinal materials and preparation of new discoveries, and had not been approed for sale in the domestic and foreign chemical drug substances and their preparations, biological products registered applicants, may apply in new drug clinical trials for apply for special approval.
Single drug classes have failure, such as restructuring humanized PCSK9 monoclonal antibody injection, humanized people interleukin 6 receptor monoclonal antibody injection, Idarucizumab injection, Ixekizumab injection, and in accordance with the law especially single injection didn't get priority review.
It is worth noting that the antitumor drug into priority review success rate is relatively high.
Conclusion the < < <
Overall, priority review declare failure mainly domestic enterprises. Perhaps that is because the domestic enterprises for priority review policy is, of deep understanding of the competitive products, information, overly optimistic about their product, product information is not sufficient.
Analysis of priority review approved list, can be reflected from the side for urgent clinical needs by CDE attitude towards innovation - and the high quality product on the market. |
