Medical network - on July 7th, who in shandong province development planning commission issued a "about in order to strengthen the management of clinical application of antibacterial drugs contain bacteria resistant work notice, and established the clinical application of antibacterial drugs classification management of shandong province directory (2017 edition)" (see annex 1), the clinical application of antibacterial drugs will be strict management, the purchase of medical institutions at all levels and use have made detailed provisions, such as the quantity and variety, the use of antimicrobial agents will face severe challenges.
1. Major changes in the list of antimicrobial drugs
"Notice" provisions of the "directory" as the basis of antimicrobial drugs classification management in shandong province and the minimum requirements, medical institutions can improve management level of antibacterial agents on the basis of the condition of the this institution, the variety of "level of restrictions on the use of" rise to "restrict the use of level", "limit the use of" species raised to "special use level" management, ban cut antibacterial drug management level. The level of restriction is increased level by step, which means that the actual use scope is much stricter than that of the catalog.
2. The use of primary medical institutions shall not exceed the secondary hospitals
The use of antibacterial drugs is heavily regulated at the grassroots level request basic-level medical institutions in shandong province selects the national list of essential medicines and supplemented only drugs in the directory of antimicrobial agents varieties, species number shall not exceed the secondary general hospital varieties. There should be no more than 35 kinds of antibacterial drug varieties in secondary general hospital, oral hospital and tumor hospital.
There are no more than 2 types of antimicrobial injection and oral dosage forms of the same generic name, and antibiotics with similar or similar pharmacological characteristics must not be repeated. The antimicrobial drugs of cephalin are not more than 2. Three generations and four generations of cephalosporins (including compound preparations) antibacterial drug oral dosage form no more than 5 standard, injection dosage form no more than 8 standard; There are no more than 3 drug formulations for the antibacterial agent of carbapenems. There are no more than four drug formulations of fluoroquinolones oral dosage form and injection dosage form. There are no more than 5 varieties of antifungal antifungal drugs.
Grassroots medical institutions in recent years in terms of infusion and antibiotics were strictly limited, and the overall limit of antimicrobial agents, grassroots doctors need to be more cautious in medicine.
3. The infusion of village clinics, clinics and community health service stations shall be approved by the county health and family planning administration department.
The notice also has strict rules for the basic infusion activities. Medical institutions and medical personnel should be required to strictly control the use of antibacterial drugs to prevent infection, and strictly control the proportion of antibiotics used in outpatients' intravenous infusion. In order to strengthen the management of grassroots medical institutions clinical application of antibacterial drugs, village clinics, clinics and community health service stations use of antimicrobial agents to carry out activities by intravenous infusion, to the family planning administrative department of public health at the county level for approval.
Iv. Special use of antibacterial drugs shall not be used in the clinic
Strictly control the use of special antibacterial drugs. Special use of antibacterial drugs should not be used in the clinic. Because such as emergency rescue critically ill patients, without the consent of the consultation or above is really necessary to use antimicrobial drugs, prescription amount shall not exceed 1, dosage, drug and detailed record against, within 24 hours above fill do formalities necessary for use of antimicrobial agents. As there are more and more restrictions, be careful not to overdose.
In accordance with the "notice", including shu ba jotham, cefepime ammonia QuNa, beauty e.faecalis, imine south/west he ding, than APei south, ertapenem, instead of add ring element, vancomycin, vancomycin, their stead ning, colistin (injection), polymyxin B,, west to acid, rina thiazole amine, doxycycline, amphotericin B (containing fat), voriconazole (injection), itraconazole (injection), mooring Finn card net, net micah Finn, a total of 20 kinds of drugs are included in the outpatient service is disabled
Text:
Notice of shandong provincial health and family planning commission on strengthening clinical application management of antimicrobial agents to control bacterial resistance
Lu wei medical word [2017] no. 26
The municipal health and family planning commission (NHFPC) is a medical institution.
In order to further strengthen the management of clinical application of antibacterial drugs in our province, improve the level of rational use of antimicrobial drugs, contain bacteria resistant, according to "about printing contain bacteria resistant national action plan (2016-2020), the notice of health medical hair [2016] no. 43 (countries) and" about further strengthen the management of clinical application of antibacterial drugs contain bacteria resistant notice (countries who do hair [2017] no. 10) requirements, by experts adjustment made the catalogue of clinical application of antibacterial drugs classification management of shandong province (2017 edition) "(hereinafter referred to as the" directory ", see appendix 1), and hereby printed and distributed to you, and the management of clinical application of antibacterial drugs, contain bacteria resistant work put forward the following requirements, please implement seriously.
I attach great importance to the clinical application management of antimicrobial agents
Currently, bacterial resistance has become a major challenge in the global public health field, and it is also a worldwide problem which is widely concerned by governments and society. At the G20 hangzhou summit in 2016, the issue of bacterial resistance was included in the main agenda, and the final communique was written. At the 71st session of the United Nations general assembly, the world discussed the problem of drug resistance and became the fourth health issue to be discussed by the general assembly. The problem of bacterial resistance has expanded from health to political and economic fields. The family planning administrative department of public health and medical institutions at all levels should start from the overall situation, from the perspective of promoting the construction of health of China, shandong, attaches great importance to the clinical application of antibacterial drugs management, further completes the related work.
2. Strictly implement the related requirements of clinical application management of antimicrobial agents
(1) adjust the list of antimicrobial drugs in time. The entire province various medical institutions at all levels should be controlled "directory", to adjust, making the agency of antibacterial drug supply directory, directory should include purchasing varieties of antibacterial agents, rules and other information. The directory for the graded management of antibacterial drugs in our province and minimum requirements, based on medical institutions according to particular case this institution improve the management level of antimicrobial agents, the variety of "level of restrictions on the use of" rise to "restrict the use of level", "limit the use of" species raised to "special use level" management, ban cut antibacterial drug management level. Medical institutions for antibacterial drug supply catalog for dynamic management, the safety, efficacy repel and uncertain, severe drug resistance, and ratio of illegal use of antibacterial drug varieties and product rules. Should be regularly adjusted according to regulations of antimicrobial drug supply catalog, adjust the cycle for 2 years in principle, the shortest not less than 1 year, and in the adjustment, within 15 working days after issue the "practice license of medical institution" of the family planning administrative department of public health for the record.
(2) strictly control the number of varieties of antibiotics purchased. In principle, there are no more than 50 varieties of antimicrobial drug varieties in comprehensive hospitals and children's hospitals; There are no more than 40 varieties of antimicrobial agents in maternity hospitals (including maternity and child care centers); There are no more than 35 types of antibacterial drugs in secondary general hospital, oral hospital and tumor hospital. There are no more than 10 types of antimicrobial drugs in psychiatric hospitals; Compound sulfamethoxazole, furan, penicillin G, benzathine penicillin, 5-fluorouracil can be counted in the variety. The primary medical institutions can only select the national basic drug catalogue and the varieties of antimicrobial drugs in the list of supplements in shandong province, and the number of varieties should not exceed the number of varieties of secondary general hospitals.
There are no more than 2 types of antimicrobial injection and oral dosage forms of the same generic name, and antibiotics with similar or similar pharmacological characteristics must not be repeated. The antimicrobial drugs of cephalin are not more than 2. Three generations and four generations of cephalosporins (including compound preparations) antibacterial drug oral dosage form no more than 5 standard, injection dosage form no more than 8 standard; There are no more than 3 drug formulations for the antibacterial agent of carbapenems. There are no more than four drug formulations of fluoroquinolones oral dosage form and injection dosage form. There are no more than 5 varieties of antifungal antifungal drugs.
(iii) strictly restrict the selection and quantity of antimicrobial agents. Medical institutions shall not purchase antibacterial drug varieties and regulations of the pharmaceutical supply of the agency. In case of special treatment needs, the use of antibacterial drugs, other than those of the agency, can be used to activate the temporary procurement procedure. The application of the same generic name of antimicrobial drug to start the provisional procurement procedure should not exceed 5 cases per year in principle. If more than 5 cases are discussed, it will be discussed whether to include the list of antimicrobial agents in the agency. The total number of total varieties of the modified antimicrobial drug supply catalog should not increase. The medical institution shall record the provisional purchase situation every six months to the health and family planning administrative department of the medical institution.
(4) strengthen the administration of prescribing of physicians. Antimicrobial agents of different management levels ChuFangQuan strictly limited, physicians use antimicrobial prescription authority at all levels, and take effective measures to ensure the implementation of the graded management system, a physician and violation went a prescription.
Iii. Strengthen management of key links in clinical application of antimicrobial agents
(a) to strengthen the management of prominent issues such as prevention of use, joint use and intravenous infusion of antibiotics in outpatient patients. Medical institutions and medical personnel should strictly control the use of antibacterial drugs to prevent infection, and strictly control the proportion of antibiotics used in outpatients. In order to strengthen the management of grassroots medical institutions clinical application of antibacterial drugs, village clinics, clinics and community health service stations use of antimicrobial agents to carry out activities by intravenous infusion, to the family planning administrative department of public health at the county level for approval.
(2) to strengthen the monitoring and management of the clinical use of antibacterial drugs in key departments. Large medical institutions in the clinical use of antimicrobial agents, use level is high, easy to cause problems of intensive care unit (ICU), neonatal room, onset ward, respiratory ward, neurological ward, burn ward and other departments and grassroots medical institutions, to focus on in order to strengthen the management of antimicrobial agents, organize regular doctor's advice for antimicrobial prescription, relevant experts focus on special spot. The problems found in the comments should be tracked, managed and intervened to achieve continuous improvement, and the results of the review should be regarded as an important basis for the awarding of the authority of the department and medical personnel and the performance appraisal. Doctors who have more than 3 over-prescription of antibacterial drugs and have no legitimate reason to warn them, limit their special use levels and limit the use of class antibacterial drugs. After restricting the right of prescription, there is still a supernormal prescription and there is no justification for the cancellation of the prescription of antimicrobial drugs, and no recovery shall be made within 6 months.
(3) strictly control the use of special antibacterial drugs. Special use of antibacterial drugs should not be used in the clinic. The rate of microbiological delivery of antimicrobial agents was not less than 80% before the use of antimicrobial agents in patients treated with special antibacterial drugs. Clinical application of special drug use level of antimicrobial agents should be controlled strictly against, the clinical application of antimicrobial drugs experience of infection or relevant professional experts consultation, by physicians has a senior professional technical position qualifications prescribed or orders rear can use. Because such as emergency rescue critically ill patients, without the consent of the consultation or above is really necessary to use antimicrobial drugs, prescription amount shall not exceed 1, dosage, drug and detailed record against, within 24 hours above fill do formalities necessary for use of antimicrobial agents.
(4) strengthen the management of antibacterial agents of carbon penicillienes and the management of tiapin. The antibacterial agent of carbon penicilliene and the special management of tiagin. In the clinical departments, the use of carbapene-like antibacterial agents and the replacement of cyclotene are required to report the information in a timely manner (see annex 2). Medical institutions to assign a regular collection, summary the agency penicillium carbon alkene antimicrobial agents and for adding ring element usage information table, and carries on the analysis, take targeted measures to effectively control the carbon penicillium alkene antimicrobial drugs and for ring resistance. Medical institutions shall report every six months will use the information to issue the "practice license of medical institution" of the family planning administrative department of public health, authorized by clinical application of antibacterial drugs monitoring quality control center is responsible for committee (tube) information collection work of medical institutions.
Iv. Improve the technical support system for clinical application management of antimicrobial agents
The family planning administrative department of public health and medical institutions at all levels should strengthen infection, clinical microbiological and clinical pharmacy discipline construction, gradually establish a diagnosis and treatment of infectious diseases, refractory disease consultation, hospital infection control, antimicrobial application management, and other related contents of diagnosis and treatment system, and in the clinical application of antibacterial drugs management play an important role. Strengthen medical personnel training, implement the national formulary, the clinical application of antibacterial drugs guidelines (2015 edition) "national antimicrobial treatment guidelines and other requirements, and the clinical application of antibacterial drugs management and work effectively in combination with clinical pathway management, clinical pathway in regulating the behavior of diagnosis and treatment, the role of work to promote rational drug use. We will increase the training of doctors, pharmacists, microbiological inspectors and managers to improve the technical and managerial capabilities of rational use of antimicrobial agents. We should strengthen the information construction of medical institutions and give play to the technical support of information technology in clinical application management of antimicrobial agents. Actively organize and carry out the propaganda work of popularisation of science popularization, create a reasonable application atmosphere of antibacterial drugs, raise public awareness of antimicrobial agents, and set up the correct concept of drug use.
5. Strengthen the clinical application of antibacterial drugs and bacterial resistance monitoring and evaluation
The administrative departments of health and family planning at all levels and medical institutions should actively carry out the long-term mechanism of the scientific management of antimicrobial drugs (AMS), and promote the continuous improvement of the management system of antimicrobial drugs. We will encourage qualified medical institutions to join the clinical application monitoring network and bacterial resistance monitoring network of antibacterial agents, and establish an early warning mechanism for the clinical application and bacterial resistance of antimicrobial agents. To timely understand or medical institutions within their respective jurisdictions in clinical application of antibacterial drugs and bacteria resistant, on a regular basis for the clinical application of antibacterial drugs management work and makes an analysis of the bacterial drug resistance situation, regularly publish monitoring and evaluation results, the use of abnormal growth, frequent usage ranking top ten and more than half of the super indications for use and frequent drug overdosage serious adverse events, and so on and so forth, investigate and take effective intervention measures in a timely manner. In the process of monitoring and evaluation, medical personnel who have found and verified against the "nine inaccuracy" and existing medical ethics problems should be dealt with according to the rules and regulations in accordance with the law.
6. Clear the clinical application management responsibility system of antimicrobial agents
The family planning administrative department of public health and medical institutions at various levels shall clear the unit and the responsibility of the clinical application of antibacterial drugs management department responsible (see annex 3), in principle, the responsibility department is mainly responsible for comrades for those responsible. The main responsible person of the medical institution is the first responsible person of clinical application management of antimicrobial agents. Information of the competent department of medical institutions and responsible persons, and the health and family administrative department of the medical institution's practice license shall be reported; Information of the responsible department and responsible person of the health and family planning administrative department shall be reported to the superior health and family planning administration department. Responsible departments and responsible persons should earnestly perform their duties, promote the rational use of antimicrobial agents, and effectively curb bacterial resistance.
7. Strengthen supervision and inspection and application of results
The family planning administrative department of public health and medical institutions at various levels shall strengthen the clinical application of antibacterial drugs for the management of daily supervision and regular supervision inspection, comprehensive medical institutions should be at least once every quarter, the family planning administrative department of public health supervision in principle, of not less than 2 times a year. We have incorporated clinical application management of antimicrobial agents into large hospitals, improved medical service operations, and the standardization of hospitals. Through supervision and inspection, continuously improve the clinical application management level of antimicrobial agents, and effectively restrain bacterial resistance. To supervise the inspection found not in accordance with the relevant requirements, the problems in the implementation of the family planning administrative department of public health, or clinical departments, medical institutions should be criticized, is mainly responsible for comrades for admonishing talk, about to cancel the qualification of a periodic review of medical institutions or the inspection result. At the same time, we should focus on the development of the clinical application of antimicrobial agents and the advanced typical and working experience of high management level, and summarize and promote the promotion.
If there are any problems or Suggestions in the implementation of the catalogue, the health and family planning administrative departments and medical institutions at all levels shall promptly report to the committee. The municipal health and family planning commission and the medical institutions of the commission will report the responsibility department and the responsible person's information (annex 3) to the medical and administrative department of the provincial health and family planning commission by the end of June. The catalogue of the supply of antimicrobial drugs adjusted by medical institutions at all levels (annex 4) shall be filed with the health and family planning administrative department of the medical institution before the end of June.
The present document shall be effective from the date of issuance to May 15, 2021. The catalogue of clinical application of antimicrobial drugs in shandong province (2012 edition) is repealed at the same time.
Shandong provincial health and family planning commission
May 16, 2017
Attachment:
1. Clinical application of antibacterial drugs in shandong province (2017 edition)
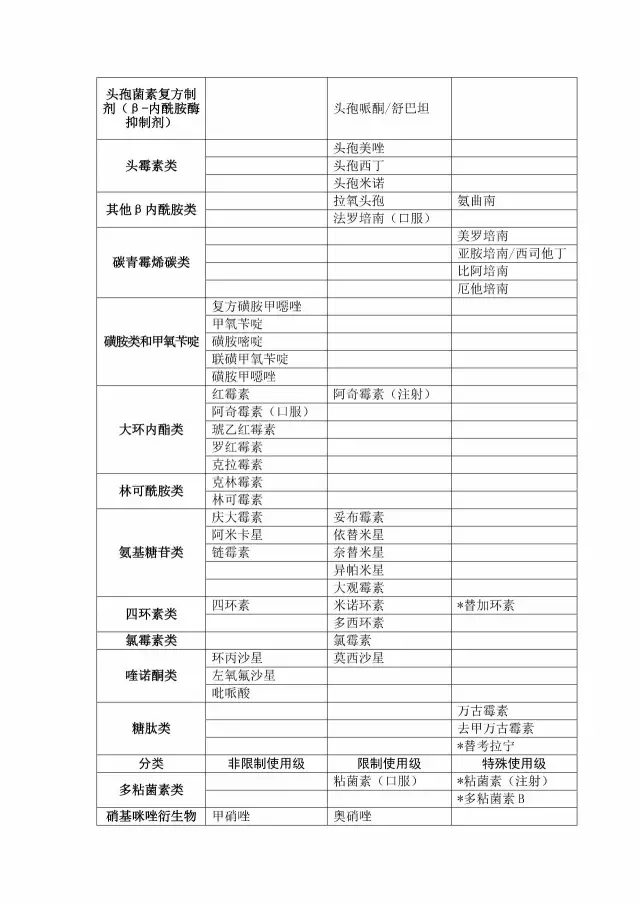 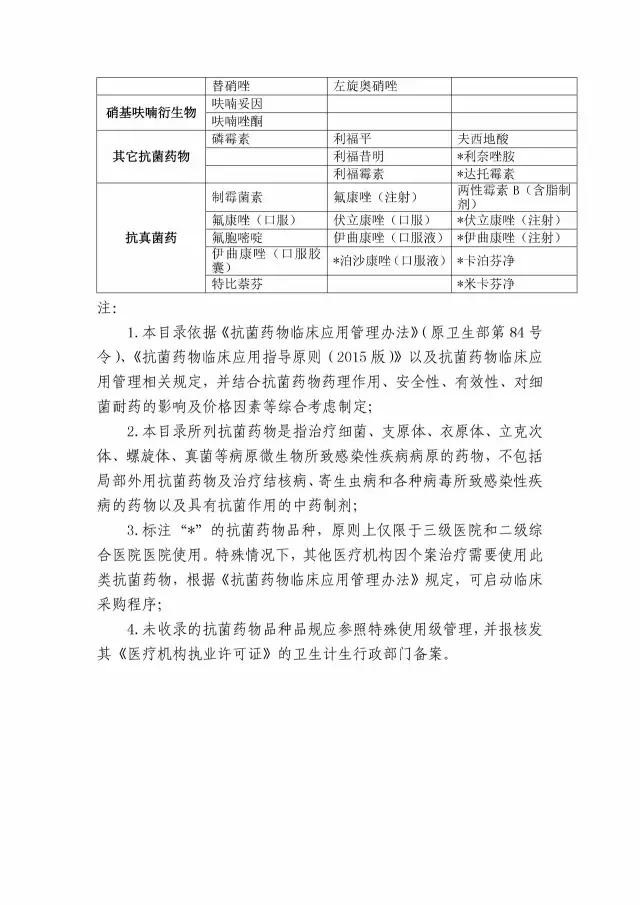
2. Information table for the use of carbon penicillienes in medical institutions and in the use of the cyclosporine
3. Clinical application of antimicrobial agents and the information table of responsible persons
4. Catalogue for the supply of antibiotics for medical institutions
|
