In the first half of 2017, the FDA approved 28 new drugs, six more than in 2016.
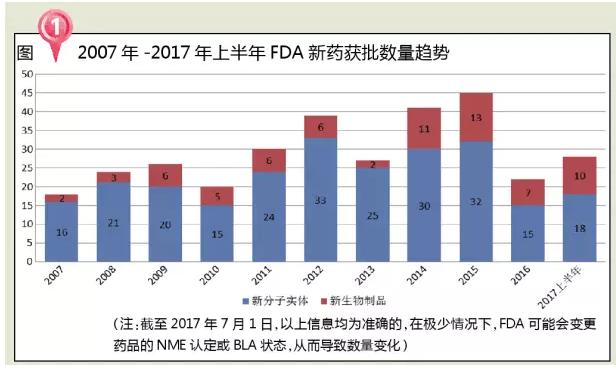 Analysis of the number of new drugs approved by the FDA in the past decade has found that the FDA approves the overall number of new drugs in the year 2007 to 2015, but the number of approved new drugs has fallen in 2016. One is that there are five NME plans approved in 2016, which are advanced to 2015. The number of applications in 2016 was relatively small and the Complete Response (CR) was more. The third is that a significant number of applications are still pending and cannot be classified as approved.
So-called CR letter, similar to the domestic supplement notice, by the FDA in writing to the applicant to send letters to complete description of the FDA found that the submission of all defects and deficiencies in the application materials, additional information in order to guide the applicant can be approved.
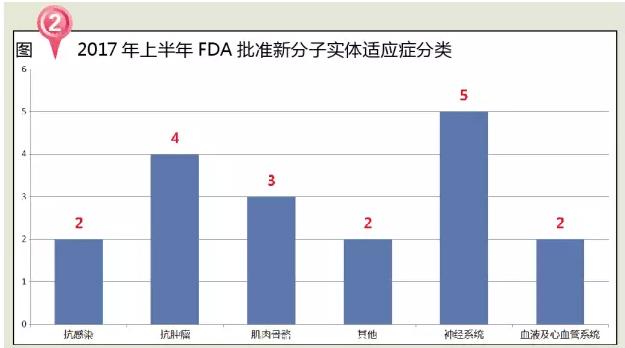
From the point of therapeutic areas, in the first half of 2017 the FDA approved the NME awards including five nervous system drugs, four antineoplastic agent, three musculoskeletal medicine, fight infection, cardiovascular system, and other drug use 2 each.
The market for nervous system drugs is one of the world's most powerful drugs. The proportion of the clinical use of the medicine terminal in China is also increasing. Nervous system drugs in our country in 2015 market size is 104.784 billion yuan, according to the Lancet and Lancet Psychiatry three papers show that people with mental illness in China accounts for about 17% of the world, but affected by resource scarcity and prejudice, the vast majority of patients has not been effective treatment. Of the 18 new molecular entities approved by the FDA in the first half of 2017, the nerve system accounted for 28% of the total. In the increasingly fierce competition in the market circumstances, the nervous system drugs market is a very good profit for the point, from the enterprise level, how to occupy a huge market is also facing a problem.
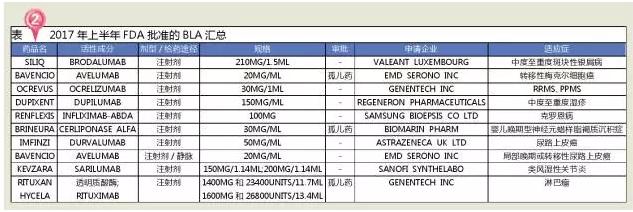
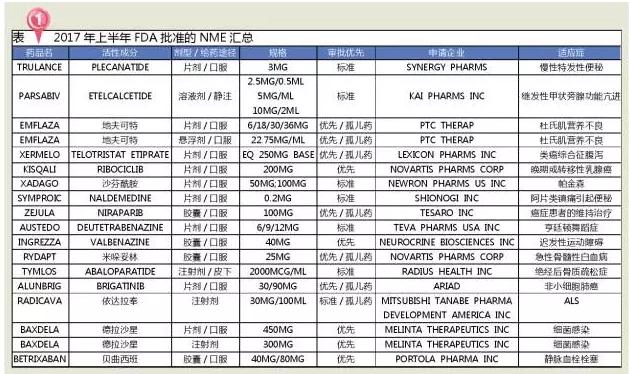 10 biological products
Nine innovative drugs, one biosimilars
In the first half of 2017, the FDA approved 10 biological agents, including 9 innovative drugs, and RENFLEXIS, developed by SAMSUNG BIOEPSIS, is a biosimilars.
IMS Health statistics show that in 2014 the global biological medicine market scale has reached $214 billion, from 10.5% in 2001 to increase market share to 21.3% in 2014, faster than the global pharmaceutical market growth the good momentum of vigorous development.
In recent years, breakthroughs in technology have also accelerated the application of biotechnology in the pharmaceutical industry and the development of new drugs. Against this backdrop, the world's pharmaceutical giants have zeroed in on the nascent field of biopharmaceuticals, vying to develop the biomedical market.
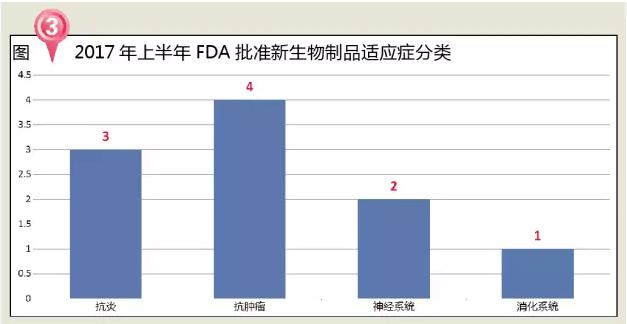 In terms of treatment, the BLA approved by the FDA in the first half of 2017 includes four anti-tumor drugs, two anti-inflammatories, two neurologic drugs and one digestive system.
 Tumor therapy is undoubtedly the most promising field for innovative drugs. In 2015, the global market for anti-cancer drugs exceeded $100 billion, with compound growth of around 6.5 percent and the potential to increase to $150 billion by 2020, according to the analysis agency. Since 1997 the FDA approved the first targeted cancer drugs Rituxan (Rituxan), the FDA opens the tumor treatment for examination and approval of the new age, the FDA in recent years, but also focus on innovative drug approval in the field of anti-tumor drugs.
Six potential "bombshells"
Three varieties forecast sales of more than $2 billion
According to EvaluatePharma drug sales forecast data in 2022, more than the first half of 2017 the FDA approved drugs will hopefully become a blockbuster drugs, the treatment of dermatitis Ocrevus Dupixent, treatment of multiple sclerosis and Durvalumab and other biological agents for the treatment of bladder cancer is expected to exceed 2 billion sales mark.
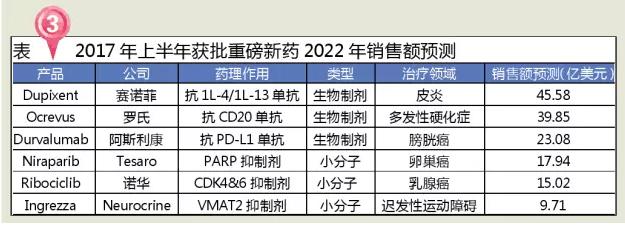 Dupixent sanofi Regeneron with the United States, which is used in the biological medicine company research and development of adult patients in the treatment of moderate to severe eczema injection, is the first and only an approved treatment of moderately severe Atopic Dermatitis (Atopic Dermatitis, AD) of biological agents. Atopic dermatitis (AD) is a chronic, recurrent, inflammatory skin diseases, related to many factors such as heredity, immune, and environment, more than infants and young children period, as the growth of the age, its a significantly increased risk of allergic rhinitis and asthma. In the United States, about 300,000 people in moderate to severely uncontrolled atopic dermatitis need innovative treatment to improve their condition. Dupixent can simultaneously blocking cytokines IL - 13 and IL - 4 signaling pathways, the signal path is due to the excessive activation of atopic dermatitis (eczema), the main cause of diseases such as asthma, according to the forecast, Dupixent is expected to become a changes dermatitis disease related drugs for the treatment of rules.
Ocrevus genentech is bought by roche (2009), which is used in the research of recurrence of multiple sclerosis (RRMS) and primary progressive multiple sclerosis (PPMS) injection, is the first PPMS drugs approved by FDA, CD20 antigen is a B cell inhibitors monoclonal antibodies. Evaluate Pharma, a medical market research firm, predicts that Ocrevus will have $2.7 billion in annual sales by 2020. Multiple sclerosis, multiple sclerosis, MS) is a chronic, inflammatory, demyelinating central nervous system diseases, can cause various symptoms, including feelings change, visual impairment, muscle weakness, depression, etc., the existing treatment can prevent recurrence of the disease, but can't reverse the pathological conditions. It is mainly divided into four categories: relapse mitigation MS (RRMS), secondary progressive MS (SPMS), original progressive MS (PPMS), and recurrent progressive MS (PRMS). Most patients are RRMS.
Imfinzi (Durvalumab) for astrazeneca and development of PD - L1 antibody, on May 3, 2017, the FDA's accelerated approval for the treatment of locally advanced or metastatic epithelial carcinoma of the urinary tract, suitable for with platinum-based chemotherapy drugs or after chemotherapy patients with disease progression, or preoperative surgery with platinum-based chemotherapy drugs in the 12 months in patients with disease progression. Durvalumab's listing means that there are currently five pd-1 / pd-l1 antibody drugs in the market, with the exception of Durvalumab, which also includes: Keytruda of Merck, Opdivo of BMS, Tecentriq of roche, and Bavencio of Pfizer. Durvalumab's most common adverse reactions (15 percent or more) were fatigue, musculoskeletal pain, constipation, loss of appetite, nausea, peripheral edema, and urinary tract infections.
Zejula (Niraparib) was developed by American Tesaro used to receive platinum drugs completely after treatment response or partial response and relapse of adult ovarian epithelial carcinoma, carcinoma of fallopian tubes and maintenance therapy in patients with primary peritoneal carcinoma of poly (ADP ribose polymerase (PARP) inhibitors. Zejula is the third PARP inhibitor approved by the FDA (the other two are Lynparza in 2014 AstraZeneca, and Rubraca in 2016 Clovis) and the first PARP inhibitor for maintenance therapy. Globally, there are nearly 1.7 million cases. 1% to 5% of breast cancer cases are due to genetic mutations in BRCA1 or BRCA2. A in Nature Medicine published the article reveals the possibility of up to 20% of women treated with PARP inhibitors, PARP inhibitors is a previously considered only women with BRCA1 or BRCA2 mutations of genetic Medicine.
Kisqali (Ribociclib) is a Noel r&d for postmenopausal hormone receptor and human epidermal growth factor receptor 2 negative + / HER2 - (HR) of patients with advanced or metastatic breast cancer women agents, is approved by FDA in the second paragraph CDK4/6 inhibitors (Pfizer Ibrance) in February 2015. Breast cancer is the second most common cancer in American women. In 2017, according to the American cancer society estimates that about 250000 more women will be diagnosed with invasive breast cancer, and as much as one-third of patients with early breast cancer will be later developed into the stage of metastatic disease. Kisqali Ⅲ period clinical research MONALEESA - 2, according to data and to letrozole (an aromatase inhibitors) monotherapy compared Kisqali letrozole make joint disease progression or death risk significantly reduced by 44%.
Ingrezza (valbenazine or NBI - 98854) is a new kind of selective vesicle monoamine transporter 2 (VMAT2) inhibitor, is approved by FDA first used in the treatment of adult tardive dyskinesia (TD) drugs, and VMAT1, DA receptor, 5 - HT receptor affinity is low, can reduce the risk theory of related adverse events, but also with antipsychotics and antidepressants. The U.S. FDA awarded breakthrough treatment and priority approval. Tardive dyskinesia is a central nervous system disease characterized by involuntary repetitive movement of the trunk, limb, and face. This is often due to other drugs, such as schizophrenia, bipolar personality disorder with the treatment of depression, psychiatric drugs inhibits dopamine receptors in the brain, which can lead to some dopamine signaling pathways of turbulence in the human body. If these disorders occur in areas of the brain that control movement, they may cause symptoms of delayed motor dysfunction. It is worth mentioning that INGREZZA and tiva's Austedo (deuterium dibutylphenazine) structure is very similar, but INGREZZA does not have Austedo's depression and a black box warning of suicidal thoughts. The pre-emptive launch of INGREZZA is clearly a bad news for Austedo.
Summary < < <
2017 is a year to look forward to, and with Mr Trump becoming President, the us pharmaceutical industry will see important changes. The "public health porter" the FDA's boss also complete the change, on May 11, 2017, the controversial the FDA's new chief executive Scott Gottlieb took office, medical and drug regulatory policy there will be major changes, the FDA recently citing illegal abuse of opioid analgesics opana ER the market actively, and launch a generic version of priority review is a very clear signal. In the face of the potential volatility of FDA regulatory oversight, it will be another century for drug companies to seize the moment. (author's unit: dongguan jinan university research institute international drug registration platform)
|
