On August 2, the medical network passed the national negotiation mechanism to make new drugs and special drugs enter the national health care catalogue, which would benefit the social security agencies, enterprises and families. Under the first and second batch of drug price negotiations of the national health and family planning commission, 39 drugs have been made.
With the continuous improvement of the regulations and concepts of new drug examination and approval system in our country, the innovative drugs that have been listed in recent years have made some breakthroughs. In the context of the increasing difficulty of new drug approvals, innovative drugs have become the most important varieties in the market. Under the strong support of national policies, new drugs developed independently, with independent intellectual property rights and an innovative drug project listed in the state major special projects, gradually entered the "early harvest period".
Countries will curative effect, the burden heavier, urgent clinical needs of variety on negotiation mechanism and enter the catalog of national health insurance b varieties, including the domestic independent research and development of more than 10 biological engineering, chemical synthetic drug innovation. After entering the catalog of national medical insurance, got a chance to occupy the market, sales will get corresponding increase, thus forming a certain economic scale, open a green channel for enterprises bigger and stronger.
A variety of sound
Varieties from the first published in 2016 price negotiations, and in July, the second batch through negotiation mechanism into the catalog of national medical insurance b varieties, including in the completed beads for single resistance, ek, compaq heap, path for, human recombinant endostatin, restructuring of urokinase, recombinant human brain natriuretic peptide, west of the amine, morpholine indications and sand jotham ester, 10 varieties for national innovation class of drugs, only 2005 years development of booster injection for the treatment of cerebral infarction injection yuri orlov kling (kay leacom) has not yet been tuning.
The 10 successful drugs were analyzed, with the price of negotiation falling by 30.50% on average compared with the lowest price of the bid, with an average drop of 44% compared with the second batch of drugs, and still a dividend of 13.5 percentage points.
According to industry insiders, an innovative drug will enter national health insurance, take advantage of new drug protection and market advance, and basically win 50% of the market share in the field. As long as you can into the medical insurance directory, mean, can recover the r&d investment can enjoy to liquidate the progress of science and technology achievements, thus to industrial upgrading first bucket of gold, change enterprise development innovation of the dilemma.
The new drug is in full view
According to incomplete statistics, there are 20 countries innovation class of drugs into the catalog of national health care drugs, these achievements inspired tasly, fosun medicine, hengrui pharmaceutical medicine, the first sound, green leaves, "the first tier enterprise to innovative drug r&d advance wave upon wave.
According to m Intranet Chinese proprietary Chinese medicine and chemical medicine hospital monitoring and analysis system (HDM), according to data from 2016 key cities for the domestic public hospital, have medical insurance directory of clinical application of the national first class of new drugs have 30 drugs and drug use amount is 4.5 billion yuan, increased by 17.29% year-on-year. The top 10 drugs were mouse nerve growth factor, butyl phthalide, recombinant human interleukin - 11, restructuring Ⅱ tumor necrosis factor, ek, slice, glycyrrhizic acid magnesium, adefovir ester, bicyclol tablet, recombinant human interleukin - 2 and bead sheet resistance. A new class of drugs that are expected to enter health insurance in 2018 will exceed the market size of 20 billion yuan.
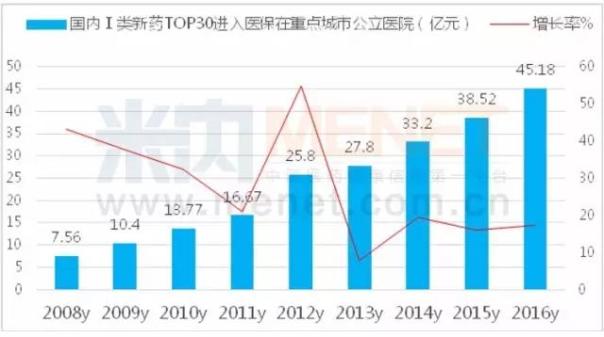 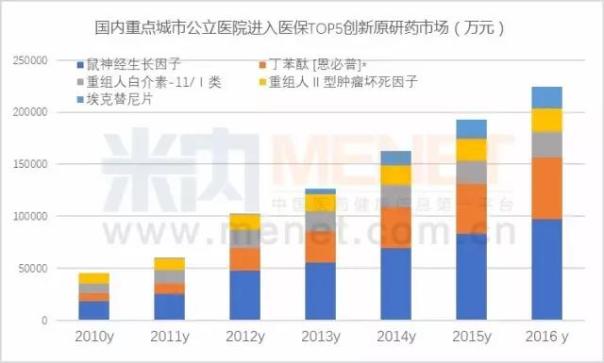 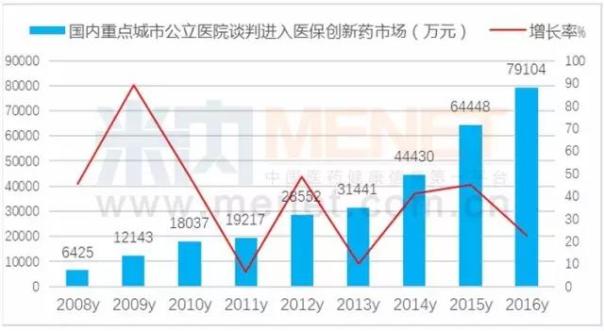 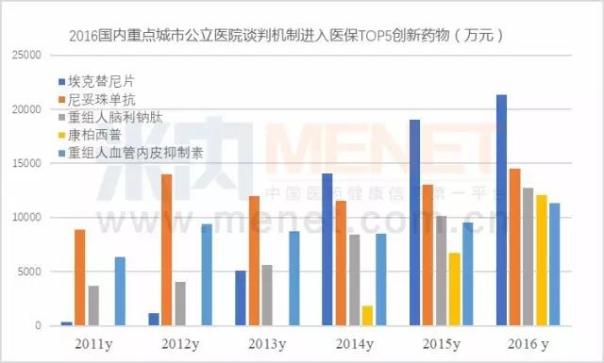 The increase in the number of domestically produced innovative drugs shows the state's strong support for homegrown innovative drugs. From the attached table, it can be seen that the reduction in price is the most important in the restructuring of human brain natriuretic peptide; Its decline was 42.02%; In Shanghai, the recombinant human urokinase price increased by 6.43%. In fact, after entering the health care system gives a person the daydream space, a large variety of negotiation on national health care by reimbursement to promote capacity growth, so as to promote the rapid growth of domestic new drugs to market.
|
