Medical network on August 9 - due to the lack of children decongestion, many children in the medicine are being treated as a "miniature adults", according to the principle of "reduction" pediatrics give children to use the adult medicine, drug use by snapping, dose by guess. In order to solve the problem of children's safe drug use, we need to mobilize the social parties to take advantage of their respective functions, so that children can use children's medicine.
About 30,000 children a year are deaf in China because of improper use of medicines, according to a new report on the safety of children's drug safety survey released in 2016. The figures suggest that children's misuse is not only harmful to children, but also to families, which is a burden to society.
Recently, in the Chinese medical association children's medication safety branch information and hosted by the Beijing children's hospital affiliated to the capital university of medical sciences, 2017 children on drug safety peak BBS, experts point out that, due to the lack of children decongestion, many children in the medicine are being treated as a "smaller versions of adult", according to the principle of "reduction" pediatrics give children adult medicine use, reducing drug use by snapping, dose by guess. "There is a huge safety hazard, and children use children's medicine."
Fewer than 2% of children's exclusive medicines
Recently, a reporter visited a number of pharmacies in Beijing and found that many of the most common pharmaceutical products on the market were no children's dosage forms, and children's special medicines were scarce. Ms. Liu told reporters that her daughter, who is three years old, went to the hospital every time to see a doctor, and went home to do the math. "The doctor says the child takes medicine to reduce the quantity, generally is an adult medicine to divide several times to eat. The larger pill parents use their hands. If the pill is small, you need to crush the pill with a tool, and then estimate the dosage to allow the child to take it under warm water. Compared with adult drugs, children use very little, liu said.
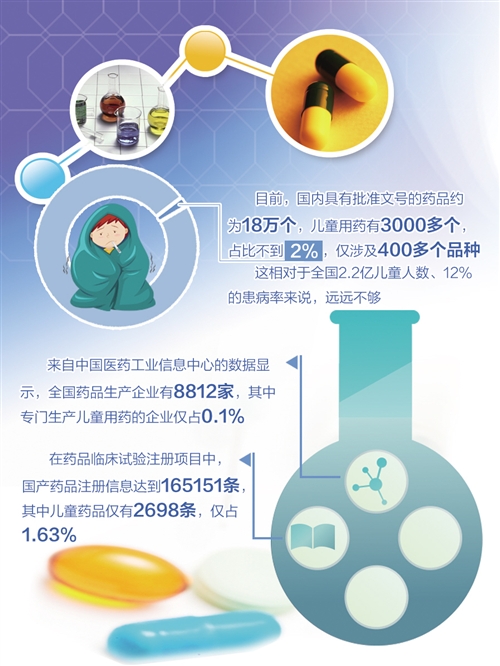
Tiantan hospital affiliated to the capital university of medical sciences, medicine, Beijing hospital authority always pharmacists frank zhao, director of the division, said that at present domestic has the approval number of drug is about 180000, there are more than 3000, children's drugs accounted for less than 2%, only involving more than 400 varieties. That's not enough for the country's 220 million children and 12 percent of the population. Suitable dosage forms and specifications for children are very scarce, especially for young children and newborns. Affiliated to the capital university of medical sciences, Beijing children's hospital, according to a survey of 231 children used prescription drugs, dosage form in the first three injection, tablet and oral liquid, which is suitable for children of dosage forms, such as powder inhaler, suppositories, syrup is very little.
Because children's metabolism, regulate ability is poor, the drug sensitivity is higher than adults, but a lot of medicine and a lack of usage, dosage, children children less decongestion, children with a greater incidence of drug adverse reaction in our country. According to zhao, the rate of adverse drug reactions in China is 12.9 percent, while the number of newborns is 24.4 percent, 2 times and 4 times higher than in adults. National food drug safety administration of adverse drug reaction, according to a report released recently 2016 report the number of children under 14 years old (including) accounted for 10.6% of total report, including severe adverse reactions accounted for 5.5% of total children report report.
Drugs are being developed into small businesses
Why are there so few special medicines for children? One is the lack of a coherent system for regulating and using the policies, said bian zhenjia, a special research fellow at the state council. On children's drugs listed in food for examination and approval of the agency, policy management in the health sector, clinical use in medical institutions, reserve management in the ministry departments, each responsible for a while, regulations, standards don't match, not unified, have a disconnect. The second is the risk of clinical trial process, which affects the clinical development of new drugs. It is also an international problem for children's drug production industry. In developed countries, the precondition for clinical trials is to sign informed consent forms with the volunteers, insure them against accident insurance and the necessary financial compensation, and the biggest expenditure on research and development is here. Third, the market of children's drug use is not high, the drug companies profit low, the production enthusiasm is not high. At present, the number of children in China is roughly stable with annual medical treatment, and the size of the market of children's drug market is not large. Children not adults, a smaller version of the organ is not mature, the clinical characteristic and the process mechanism of drug absorption, compliance, need special formula, preparation and taste, makes the drug research and development production process, the cost is high, more leading companies reluctant to engage in the r&d, production and sale of children decongestion.
According to data from the China pharmaceutical industry information center, there are 8,812 pharmaceutical companies in China, with only 0.1 percent of the companies specializing in the production of medicines for children. In the registration of drug clinical trials, the registered information of domestic drugs reached 165,151, of which only 2,698 were children's medicine, accounting for 1.63%. Only 2.35 per cent of children's medicines are registered in clinical trials, compared with more than 20 per cent in developed countries such as the us.
"Because of the children's medicine pricing principle is how much medicine effective component content as a benchmark, children is far lower than the adult medicine medicine effective component content, the corresponding price will be lower than adult medicine, make children medicine does not have obvious advantages in price, enterprise can't be compensated." Zhejiang huahai pharmaceutical co., LTD., general manager of bao-hua Chen says, to resolve the shortage of the children's medicine kinds of problems, can be made by a professional association experts summarizes the epidemiological situation and domestic present situation of children's drugs, determine which pediatric diseases need to overcome lack of drugs, and according to the pediatric medication guide and clinical experience, both at home and abroad for encourage the development of children's drugs directory, a priority review for examination and approval, guide the research and development of enterprises.
In addition, relevant experts in the study found that domestic children's drug manuals generally lack pharmacokinetic data, even if there are many foreign children data. In some domestic drug instructions, the children's clinical trial data are basically copied and imported into the original pharmaceutical instruction manual. Relevant experts say, children and adults, children between different countries there are differences between the generation of data, need China children's clinical trial data, to make them more accurate, but clinical data did not go far enough in our country.
Encourage the development of children's medicines
"In order to protect the health rights of children and encourage the development of children's medicine. In recent years, various departments have formulated policies on the protection of children's medicines. National health drug administration department, deputy director of the state family planning commission Zhang Feng said that since last year who development planning commission, ministry of industry and food drug safety administration announced two batches of encourage r&d declare children drug list, a total of 71 varieties, and clearly the "listing" declaration review of drugs by establishing in special channel, in Hong Kong, Macao and Taiwan regions have been used for years, clinical curative effect is good and it is safe to use, but not listed on the mainland children need drugs, explore import using pilot, in the drug registration review import children allow direct references to Hong Kong, Macao and Taiwan area children clinical medication data as a declaration of the basis, to speed up the review for approval. In addition, through the national "major drug discovery" major projects of science and technology, biological medicine and vaccine major innovation and development of protein engineering, integration of collaborative innovation advantage unit, guide and encourage enterprises to research and development production. In terms of guaranteeing the production and supply, we give policy support to the corresponding production enterprises of the corresponding children, and promote product upgrading and technological transformation of production lines.
Wang tianhe, secretary of the party committee of Beijing children's hospital, said that the clinical trials of children's medicine faced difficulties such as difficulty in evaluation, high risk of evaluation, difficulty in recruitment and lack of professional talents. Will be in Beijing children's hospital during the period of "much starker choices-and graver consequences-in" combined several units, further strengthening the construction of children's demonstration new medicine clinical evaluation support system, through the children's drug clinical trial optimization design key technology platform construction, and key techniques of medicines for children big data clinical evaluation platform construction, improve the ability of clinical evaluation of medicines for children. Three clinical specialties were selected as representative of children's common diseases, children's major diseases and children's rare diseases, and established a demonstration technology platform for clinical evaluation of children's new drugs.
Meanwhile, the deputy director of the national health and family planning commission, zeng yixin, said that six departments, such as the national health and family planning commission, have issued a number of opinions on the protection of children's medicines, and have made two batches of encouraging research and development lists. In particular, in the major national special projects of new drug development, the research and development of children's medicine and the acceleration of the approval are the key tasks. "For children's medicines, appropriate doses should be given to different children; The dosage form should be oral liquid; And it should be suitable for children to drink. This is the key task of the 13th five-year plan, together with the ministry of science and technology, the ministry of finance, food and drug administration, and the health and family planning commission, to speed up the development and approval process for Chinese children. It is estimated that children's drug shortages will be effectively solved in a few years.
"The healthy growth of children is about the harmony and well-being of every family and the progress of national health and sustainable development." Zhang Feng said that children's drug safety problem of the broken, you also need to mobilize social parties to take advantage of their respective functions, including government organs, social organizations, enterprises, hospitals, media, etc., at the same time should also carry out the mass children's scientific knowledge dissemination and education, etc.
|
