| Micro-channel |
 |
|
|

|
|
|
| |
| 31 medicines will be subject to on-site verification of clinical trial data! |
| |
| Author:中國銘鉉 企劃部 Release Time:2017-8-21 10:59:53 Number Browse:692 |
| |
On August 18, 2008, the food and drug administration of the state administration of food and drug administration issued a notice of on-site verification plan for clinical trials of drugs (no. 13).
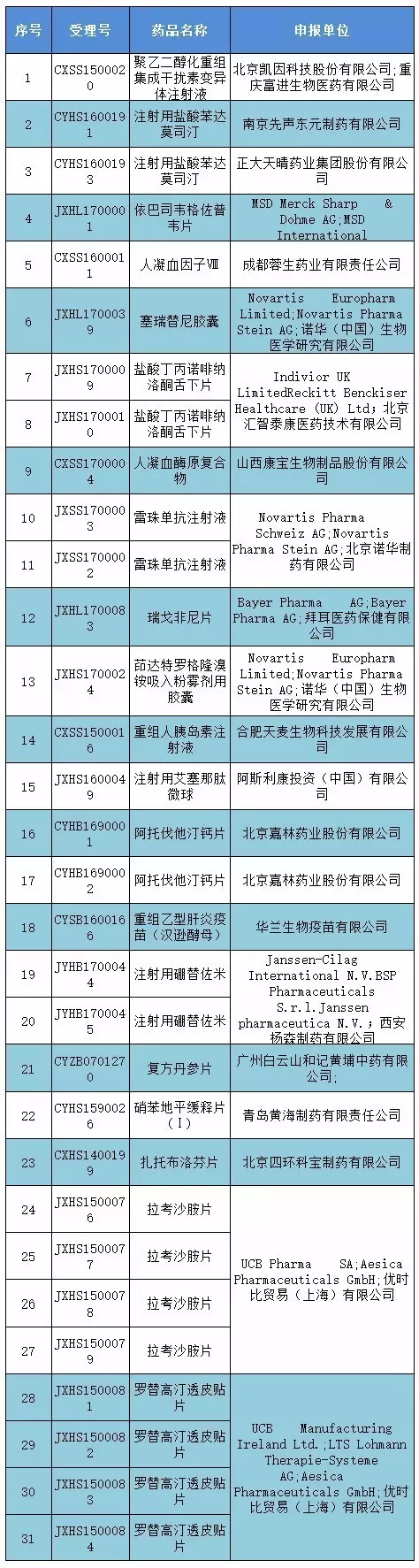
Announcement said, according to the state food and drug supervision and management of the administration of the drug clinical trial data verification procedures (provisional) "(food drug safety medicine tube [2016] no. 34), polyethylene glycol (peg) on the restructuring integration of interferon variant injection (acceptance number: CXSS1500020) and other 31 drug clinical trial data check list check varieties (see the appendix of furniture list for detail), carry out the inspection are hereby published.
The public notice period is 10 working days, namely August 18, 2017 solstice, August 31, 2017. After the official period ends, the on-site inspection shall be conducted.
Attachment: clinical trial data site verification of the catalogue
 |
| |
Previous article:The change of TOP20 in urban community since grade therapy
Next article:More than 100 drugs were removed and the drug was "injured".
|
| |
|
|
