| Micro-channel |
 |
|
|

|
|
|
| |
| Nine pharmaceutical companies have received 12 GMP certificates. |
| |
| Author:中國銘鉉 企劃部 Release Time:2017-8-22 11:14:01 Number Browse:633 |
| |
Medical net on August 22 12 GMP certificate repossessed!
▍ Shanghai to recover the first GMP certificate in 2017
On August 21, Shanghai food and drug administration issued a notice of GMP certificate (no. 1, 2017).
The GMP certificate of Shanghai huqingyutang Chinese herbal medicine co., LTD., became the first case of Shanghai food and drug administration in 2017.
Announcement shows that due to the violation of the "drug production quality management norms" (revised edition 2010) regulation, according to the measures for the administration of pharmaceutical production quality management standard certification provisions of article 33 of the Chinese medicine yinpian "medicine GMP certificate", the company was withdrawn.
On August 15, Shanghai food drug administration issued a special inspection of the implementation of the city's Chinese medicine yinpian enterprise notice, to standardize Chinese medicine yinpian management, to crack down on illegal business behavior, promote the development of Chinese medicine yinpian market norms.
Just one week, the results were immediate.
▍ yinpian problem, once be fined
The company has been fined for producing counterfeit medicines.
According to the March 8, 2017, Shanghai food drug administration issued "Shanghai food drug safety (serve) in the word [2016] no. 2120160104 on the administrative penalty", according to the "Shanghai huqingyu hall Chinese medicine yinpian co., LTD produce fake drugs (radix bupleuri) case", the company was fined 40000 yuan total.
The certificate was taken back and the company's losses were more serious than fines.
▍ 12 GMP certificate be retrieved
Besides, according to statistics, from the end of July to the present, a total of 12 GMP certificates have been recovered from nine pharmaceutical companies nationwide. Among them, chongqing saiwei pharmaceutical co., LTD. "lost" 4.
Unlike in the past, there are only 1 of the companies that have been recovered. In this way, it is a special inspection of traditional Chinese medicine, which plays a role. The certificate is retracted and will be sent back after rectification and acceptance. The process could also be quick, with the hainan food and drug administration reclaiming a pharmaceutical company's certificate on 27 July and sending it back on August 17.
Attached: the GMP certificate is recalled
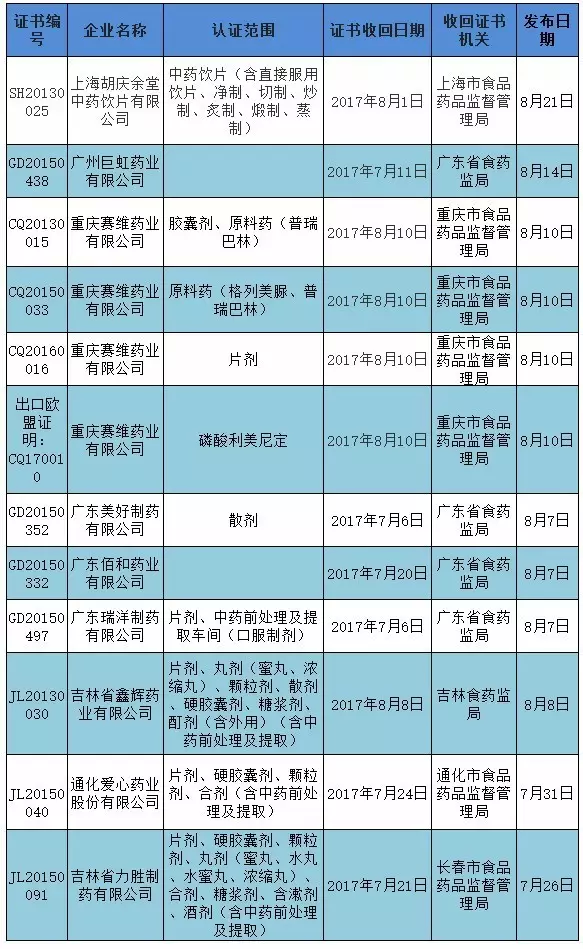 |
| |
Previous article:The threshold of the financial industry in the hundreds of billions of medical supply chain is a constraint
Next article:Drug companies have moved into pharmacies, with prescription drug sales surging
|
| |
|
|
