| Micro-channel |
 |
|
|

|
|
|
| |
| The conformity assessment official touch base: 289 catalogue 3 varieties have been abandoned, 12 varieties do not give up but no enterprises carry out evaluation |
| |
| Author:中國銘鉉 企劃部 Release Time:2017-8-23 10:37:01 Number Browse:703 |
| |
Medical network August 23 - the CFDA website issued the enterprise to carry out the quality and curative effect of generic drugs within 289 directory consistency evaluation basic situation table "(hereinafter referred to as the" table "), which is the CFDA organization provincial bureau for the enterprise to carry out the quality and curative effect of generic drugs within 289 directory consistency evaluation progress after a baseline survey, according to the report of the enterprise, the CFDA statistical summary of research results (statistics deadline: on May 23, 2017).
It is worth noting that 289 directory, medroxyprogesterone acetate capsule and diclofenac sodium sustained-release capsules (Ⅲ), three varieties of clindamycin hydrochloride by companies choose to give up temporarily consistency evaluation, the three varieties in 289 directory are only 1 companies hold approval document;Metronidazole capsules, ferrous sulfate zyban, hydrochloric acid laurel lamictal tablets, benzene azole sodium piece, morphine sulfate zyban, acetaminophen particles, ring spore capsule, lactose acid clarithromycin tablets, diclofenac sodium zyban (Ⅴ), hydrochloric acid loratadine capsule, loratadine tablet and levofloxacin hydrochloride 12 varieties enterprises choose not to give up temporarily, but also not to evaluation.
For the time being, enterprises choose to give up and choose not to give up, but not to evaluate
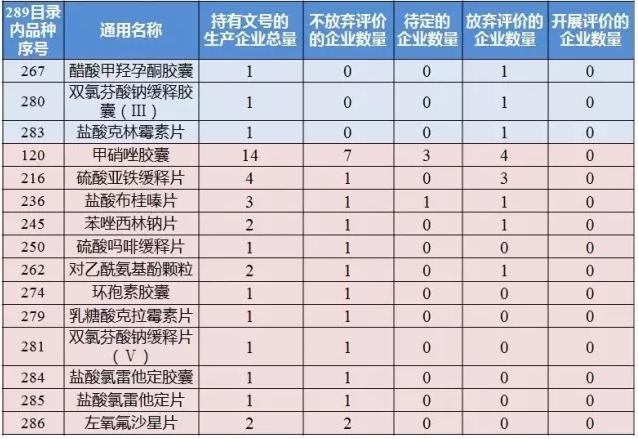
In 12 don't give up but not yet to carry out the evaluation of the varieties, except metronidazole capsules with ferrous sulfate zyban, other varieties in the 289 directory hold approval document number of manufacturing enterprises are not more than three.According to the situation table, there are 14 companies in the capsule of metronidazole, seven of which do not give up evaluation, three are to be determined, and four are temporarily choosing to give up.There are four enterprises holding the number of ferrous sulfate tablets, and the other three will give up temporarily except for one.
In addition, the conformance evaluation of compound sulfamethoxazole tablets, norfloxacin capsule and metronidazole is the most intense and the number of enterprises evaluated is more than 100.
|
| |
Previous article:The largest equipment purchase in history has shocked the waste standard, 100 million yuan product refill!
Next article:Medical website August 23
|
| |
|
|
