| Micro-channel |
 |
|
|

|
|
|
| |
| CFDA post: personal medicine approval, production and marketing release! |
| |
| Author:中國銘鉉 企劃部 Release Time:2017-8-23 11:08:52 Number Browse:1079 |
| |
Medical network - on August 23, drug research and development innovation has been the pain points of the pharmaceutical industry in our country, in recent years the state has adopted many policies to encourage drug research and development innovation, one of the most heavy system is undoubtedly the drug marketing authorisation holder pilot system (MAH), 10 provinces and cities nationwide to experimentation with...
▍ MAH has been clear about the production, the sale way
Yesterday (August 21), the CFDA encourages along blockbuster drug innovation policy of the administration of pilot work on promoting drug marketing authorisation holder system related matters notice (hereinafter referred to as "notice", see attachment for full text link), has been clear about the holder of the rights and obligations and legal liability, commissioned in the production of quality management system and production and sales of the whole chain of responsibility system, cross-regional drug regulators regulation, duty and responsibility to the ground.
The main body of the notice stipulates that:
(1) under the premise of ensuring the consistency of drug quality and efficacy, the holder is allowed to apply for a number of enterprises to produce and process the products.After receiving the first batch of production, the holder may then entrust other production enterprises to produce and manufacture the products.
(2) pharmaceutical research and development institutions and scientific researchers may sell their medicines on their own as holders, but they shall have the capacity and conditions for the operation of drugs prescribed by the drug administration law.It may also entrust a pharmaceutical producing enterprise or a drug-handling enterprise with a drug-handling license to sell drugs.When entrusted to sell drugs, a contract shall be signed to clarify their respective rights, obligations and responsibilities, to comply with relevant laws and regulations, and to implement drug traceability and quality management responsibilities.
Obviously, for research and development of Chinese pharmaceutical research and development institutions, individuals and type of drug firms have positive significance, from a deeper, more a kind of encourage innovation measures for pharmaceutical research, pharmaceutical research institutions and innovative drug firms don't have to worry about drugs do not have the production, the sale conditions, all can entrust qualified drug firms to conduct production and sales.
According to the beginning of the CFDA related files, since the middle of 2016 the state council's office issued a "drug marketing authorisation holder system pilot scheme" to the end of the year, since the released drug marketing authorisation holder declare varieties 165, mostly class 1 new drug, estimates that currently has more than 400.
▍ MAH commercial circulation industry brings new change of the pharmaceuticals
Drug marketing authorisation holder system of our country medicine industry is not only the influence of drug research and development innovation, back to our country medicine bring new change, commercial circulation for the supply side of the drugs circulation structural reform, provide policy support.
On the one hand, within the framework of drug marketing authorisation holder system pilot, part of pharmaceutical trading enterprises can actively into drug marketing authorisation holder, through the form of technical transfer or mergers and acquisitions, equity cooperation with research and development institutions, production enterprises, the use of their advantages to form a "strategic alliance", relying on professional marketing team and the control of drug sales terminal, can be implemented in a larger extent, pharmaceutical production, circulation industry chain integration, so as to obtain greater economic benefits.
, on the other hand, drug marketing authorisation holder system existing in medical market for a long time to break the "technology, industry and trade" model of development provides a possible, make the medicine circulation enterprises from passive to active, in the position in competition with the pharmaceutical circulation enterprises grasp the market dynamics and the future trends, found that most market prospects of the drug has a natural advantage, with the commerce, industry and technology development strategy to industrial chain upstream reverse development.
▍ drug firms to meet good research and development institutions, research and development type
China's new drug research and development environment has been improving, and various policy support including the system of drug listing permit holders has attracted a large number of returnees to participate in drug development and innovation.
Drug marketing authorisation holder system is for new drug research and development of "decoupling", can be said to be in the pharmaceutical technology sounded the horn has innovative new, for drug development and industrial policy solution of pharmaceutical production "bundling" to each other.
Under the circumstances of drug marketing authorisation holder system, drug firms are no longer the only holder of drug approval, fully enhance the value of the drug research and development personnel, attracting a large number of returnees entrepreneurial constantly for new drug research and development, in the current state encourages innovation, open the green channel of innovation for examination and approval of drugs such as policy, driven by innovative drug research and development and review cycle is expected to shorten, effectively prolong the product life cycle.
In addition, the medical insurance directory adjustment window open, there will be more and more domestic innovation medicine into local health insurance payment, patients pay level of ascension to further promote the sales volume growth, and to a large extent encouraged new drug research and development of passion.
I collect domestic drug firms class 1 new drug reserves in more than five three companies in product research found that: the enterprise class 1 new drug research and development on tumor, cardiovascular disease, and other fields.
Drug firms are numerous r&d type would these two diseases therapy as a research and development center, because these two kinds of diseases in the international and domestic market demand, many patients with drug treatment has yet to meet the demand, the future development prospect.
(note: the author only selected the typical three drug firms is analyzed, as companies to declare the registration information is dynamic, information sources, hard to avoid in exhaustive, only for readers reference, top drug firms on subsequent 2016 years research and development.)
Hengrui medicine, domestic tumor target 1 drug research and development flagship, China's "roche"
Many years ago, medicine circle because hengrui excellent generic drugs research and development and marketing management referred to as "China takeda", after years of development, has become a "world hengrui", China's "roche" in the field of cancer drug development.
Hengrui pharmaceutical has a r&d team of more than 2000 people, of which more than 1000 doctors, masters and more than 100 foreign employees, and in the United States, Japan and China have more research and development center, adhere to the research and development in sales by about 10% a year, in 2016, the year of drug r&d spending more than $1 billion.
At present, the company is focusing on the research direction of anti-tumor drugs, surgical anesthetic drugs, contrast agents, major diseases and the areas that have not yet been effective in the treatment of drugs, and has formed a huge product development pipeline.
In terms of innovation results, the company currently has two 1.1 class innovative drugs, erixib and apatinib.Including tumor medicine path for since December 2014 large-scale sales, maintain the accelerated growth trend, in 2016 is expected to end sales of more than 300 million yuan, non-steroidal anti-inflammatory drugs iresearch yesterday cloth is expected to have nearly one hundred million yuan.
In addition, enterprises also have a wealth of 1 kind in the research product pipeline reserve.According to the statistics of the public data, there are 17 new drugs of hengrui medicine, and 12 of them are cancer drugs, and mainly targeted drugs.
Only in the first half of 2017, there are about 80 varieties of new drugs in China, including 6 1 new drugs, which show its strong research and development strength.
Cancer drugs topped the list of the major categories of drug use in the world, and ranked among the top of the drug market in China.
According to beida pharmaceutical last listed IPO prospectus, according to the size of tumor drug market in China is close to one hundred billion yuan, including targeted drug sales has more than 12 billion yuan in 2015, the healthy knowledge jun estimated 2016 sales of the product is close to 15 billion yuan.
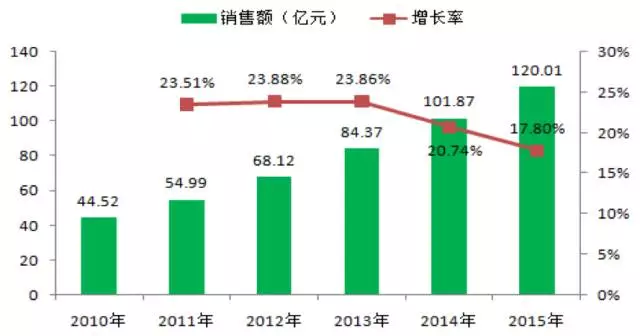
(note: data from beida pharmaceutical issue, for reference)
The product situation of the new drug of hengrui medicine:
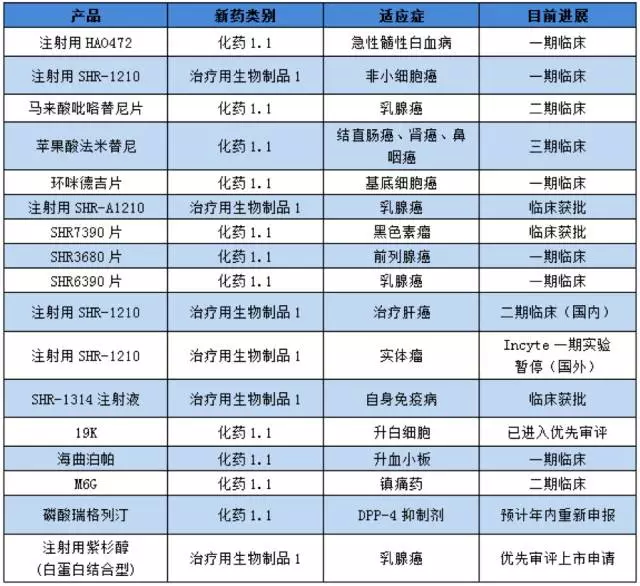
(note: based on public information, for reference)
East sunshine, the core of anti-viral drug development, China's "gilead"
The dongguang pharmaceutical research institute has performed well and benefited from the return of overseas varieties.As one of the top pharmaceutical companies in China, the group research institute has a new research and development base of new drugs, biological drugs and generic drugs.The group research institute has performed well in 2017.
In the first half of 2017, there are about 80 varieties of new drugs of domestic declared drugs, of which hengrui has declared 6 1 new drugs, showing its strong research and development strength.Dongguang group followed with five new drugs.As the only domestic preparation platform of the group, the company enjoys the right of preemption of research and development products of the group research institute, which will benefit from the rapid development of the group research institute.
Although east sun medicine also declare class 1 new drug with multiple tumors, but the company is now the dominant varieties of oseltamivir phosphate (trade name: wei) and fastest class 1 new drug research and development progress of phosphoric acid in rice he wei are antiviral drugs, so it can be a Chinese "gilead".
In the development of the product echelon perfection, will gradually face the market.The company's existing research and development pipeline echelon improvement, deep tillage against hepatitis c virus and blood sugar two areas.In the case of antiviral, the company's anti-hcv DAA drug is currently in phase II phase II clinical trials and is expected to be listed in 2020.
In addition, the company's multi-point distribution of anti-hepatitis c drug market and the promotion of the joint programme of emetavir and vlaarivir are expected to be the first domestic enterprises to introduce the all-oral treatment of interferon in China.
The specific conditions of the new drug in the study of the new drug of east sunshine medicine are as follows:
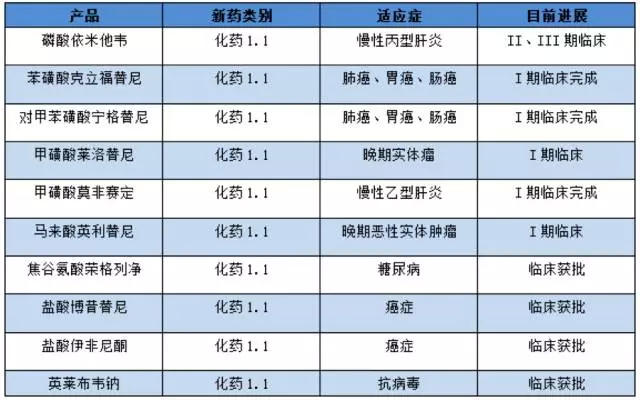
(note: based on public information, for reference)
Stone drug group, with cardiovascular disease as the main "multi-point blooming"
Shiyao group is moving from raw materials to high-end innovative drug makers.In 2012, shiyao was reorganized and its innovative drug business was incorporated into the company. Since then, the company has gradually moved towards high-end innovative drug manufacturers.
Similarly, shiyao group has a large number of new drug reserves.The company currently has about 170 products, including 15 new drugs, 105 generic drugs and 21 varieties of American ANDA.
The field of drug research and development is also multi-point, and there are 1 new kinds of new drugs in the field of chemical medicine, Chinese medicine and biological medicine.
The company is in the development of a new class of new drugs, which are the most promising varieties, with cardiovascular and cerebrovascular diseases, as well as the wide use of drugs in the field of diabetes, tumor and other diseases.
Cardiovascular and cerebrovascular disease is the largest drug use category in China, and the total market of Chinese and western medicine is estimated to exceed 150 billion yuan.With the aging of China's population, the market prospect of senile cerebrovascular disease drugs market is very promising.
According to the current research and development schedule, it is expected that the fastest listed will be the compound levorostatin, which is used for the hypertension of cardiovascular disease, and is expected to be listed in 2018-2019.
Shiyao group has entered clinical 1 new drug in the following table:
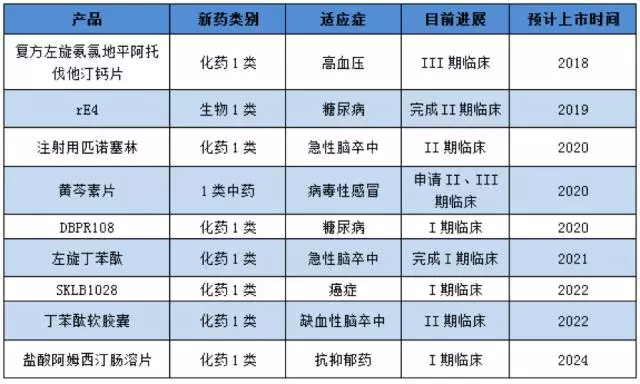
(note: based on public information, for reference)
▍ these businesses, or the biggest beneficiaries of MAH pilot system
Drug research and development innovation ability stronger drug companies or research and development institutions, due to the drug marketing authorisation holder pilot system to encourage, drug development and drug production no longer bound, innovative drug firms will greatly speed up the pace of industrialization, this kind of enterprises will be the main beneficiaries MAH pilot system.
For example, in the system within one year after release, qilu pharmaceutical co., LTD. (hainan) is to get the system in our country the first generic drugs, the treatment of and quickly realized industrialization.
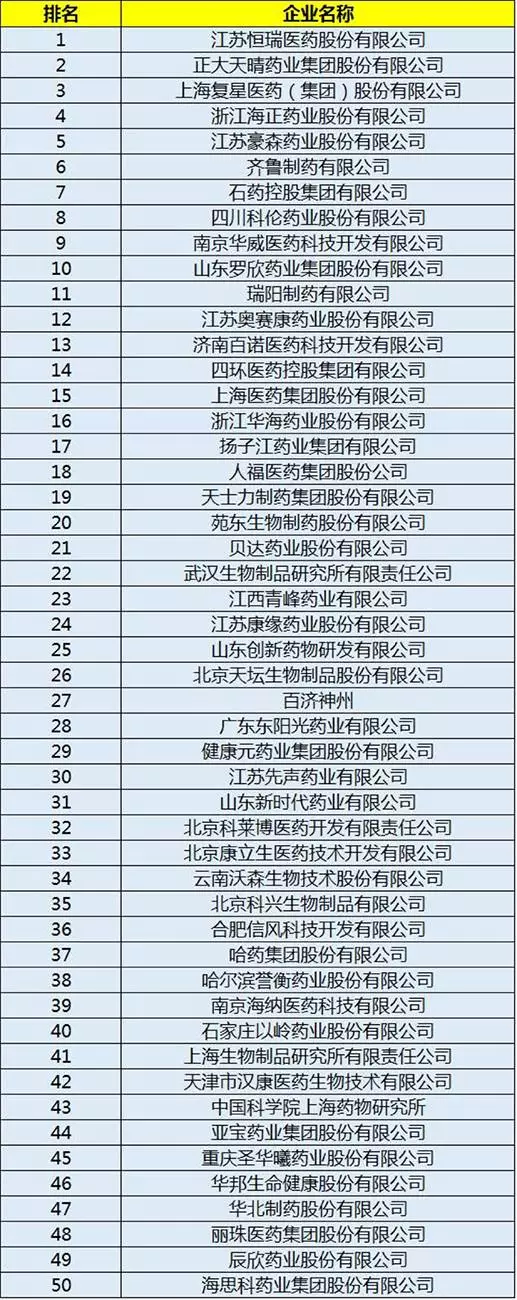
According to the China pharmaceutical university, the contemporary development research center of traditional Chinese medicine and other agencies held 2016 issued by the China research and development strength fortune 100 list, the list is not only a drug firms, as well as drug research and development institutions, these enterprises will become the biggest beneficiary MAH pilot system.
The detailed list is as follows:
|
| |
Previous article:Two ministerial posts: internal reporting rewards double up to 500,000!
Next article:CFDA: four types of medical cases focus on employee reporting bonuses double
|
| |
|
|
