| Micro-channel |
 |
|
|

|
|
|
| |
| Alishatan, a Chinese new class of new drugs, is trying to stir up the 80 billion hypertensive market |
| |
| Author:中國(guó)銘鉉 企劃部 Release Time:2017-8-24 11:27:50 Number Browse:960 |
| |
According to the 2015 China guidelines for the rational use of hypertension, the number of adults with hypertension in China has reached 298 million.On hypertension threshold, the present situation of the face is still exist gap in awareness, treatment and control, after many years of popular science knowledge propaganda and education to promote increased to 46.5% of the patients' awareness, treatment rates to 41.1%, but control is only 13.8%.
It is well known that high blood pressure is a result of genetic and environmental factors.With the rapid development of science, rapid development of sequencing technology, the genetic factors of hypertension have made great progress, which has promoted the development of anti-hypertensive drug market in China.
The 2017 edition of the national basic medical insurance, industrial injury insurance and maternity insurance drug catalog contains 271 cardiovascular drugs, of which the western medicine is 144 and the Chinese medicine is 127.It is worth noting that six of the 36 drugs in the second batch of negotiations that entered the health care directory in 2017 were cardiovascular drugs, of which alishatanate was of concern.So far, the angiotensin receptor antagonists (sartan, ARB) and compound preparations have been introduced into the health care system and have been developed to improve the structure of the anti-hypertensive market.
The market for hypertension is over 80 billion
In recent years, with high blood pressure education in our country and the improvement of medication, the majority of the elderly hypertension management had certain improvement, objectively to promote the steady growth of the treatment of hypertension in China market.However, the trend of the development of hypertensive youth has led to a significant increase in hypertension prevalence in China.
According to the data, the market size of Chinese hypertension drugs exceeded 80 billion yuan in 2016, up 13 percent year on year.Under the increasingly perfect medical security system, urban public hospitals, public hospitals at the county level, urban communities, towns and townships has become the main body of blood pressure drugs market, calcium channel blockers (CCB), angiotensin receptor blockers (ARB) and compound antihypertension drugs three sub-types constitute the tripartite confrontation.
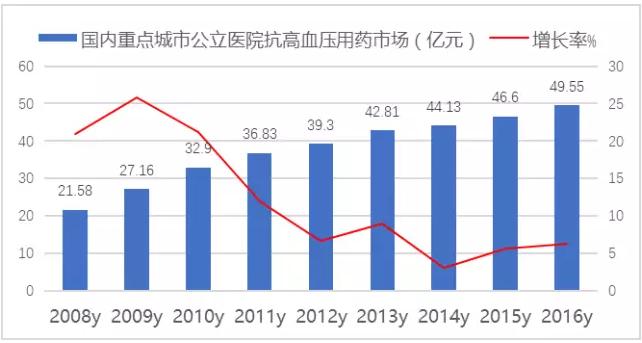
According to the data of the HDM system, the market of hypertension drugs in public hospitals in 16 key cities in China reached 49.55 billion yuan in 2016, up 6.32 percent from the previous year, accounting for 1/3 of the total cardiovascular drug use.Among them, sartan is the first, accounting for 38.25% of the proportion.
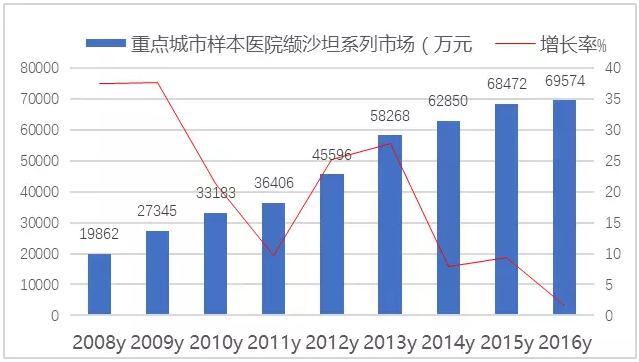
Figure 1: public hospital hypertension medication market in public hospitals in China from 2008 to 2016
"Sartan" drugs have grown steadily
Sartan drugs are antihypertensive drugs with high selectivity, high efficiency and long-term efficacy and multi-organ targeting protection, which overcomes the adverse reactions of the pulipids (ACEI).The mechanism is more specific, and the ACEI drugs have been partially replaced by ACEI drugs in the clinical medicine, resulting in the shrinkage of the growth of the ACEI market and the steady growth of the sartan drugs.
FIG. 2 market trend of six health blood pressure drugs in public hospitals in China's key cities from 2007 to 2016 (unit: 10,000 yuan)
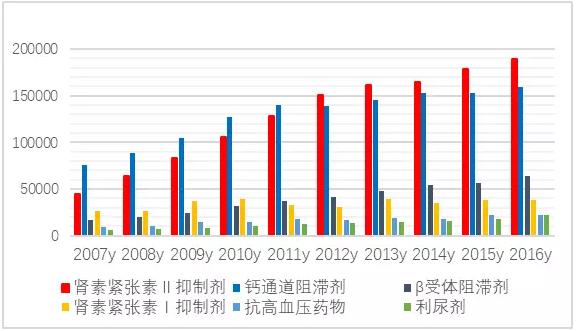
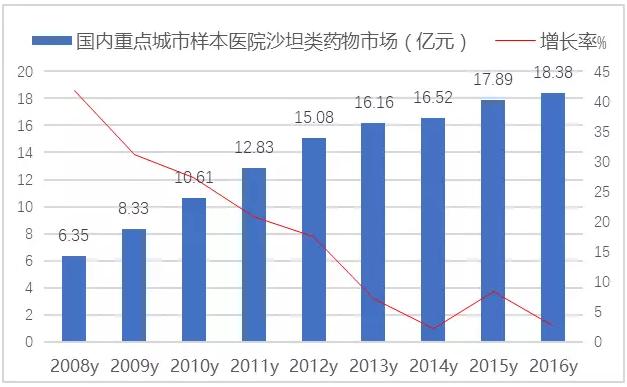
According to CFDA data, 290 copies of the approved ARB products have been approved in China.With the replacement of blood pressure drugs, the process of China's cardiovascular drug market and international market is accelerating, and ARB drugs have developed rapidly in the domestic sample hospital market.In 2016, the amount of sartan drugs and their compound preparations in 16 key cities in China was 1.838 billion yuan, up 2.70% from the previous year.
FIG. 3 the market of sartan drugs in the sample hospital of China's key cities from 2008 to 2016
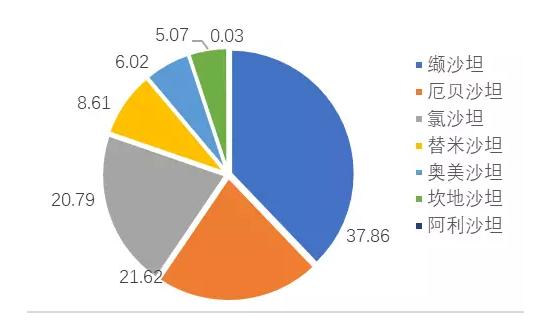
This class of drugs in addition to valsartan, potassium chloride sand, he sha Tanzania, telmisartan, ogilvy sand jotham, candesartan and seven unilateral varieties with sand, and valsartan amlodipine, hydrochlorothiazide he sand, potassium chloride sand jotham hydrochlorothiazide, valsartan hydrochlorothiazide and telmisartan hydrochlorothiazide, hydrochlorothiazide ogilvy sand, candesartan hydrochlorothiazide seven compound preparations such as varieties.The TOP 3 varieties are valsartan, urbesartan, and chlorxatan potassium, which is a new class of highly competitive hypertension drugs.
FIG. 4 competition pattern of sartan drugs in key cities in China (%)
Valsartan leads the market for hypertension
Valsartan is a kind of peptide angiotensin Ⅱ receptor inhibitor, developed by novartis, Switzerland, in 1997 the FDA approved, the goods is called Diovan.In 1998, the company's valsartan was registered in China and the commodity was called diwen.
With the preparation of the valsartan hydrochlorothiazide in novartis.The combination of the valsartan and the valsartan compound of valsartan and the preparation of the valsartan medicine has made up the negative impact on the market after the expiration of the valsartan patent, which also formed the valsartan series.
According to a 2016 novartis company annual reports, valsartan series product sales were $2.169 billion, including generation of $1.073 billion, valsartan amlodipine was $926 million, still leading global hypertension treatment drug market.
In 2016, the market for valsartan and compound preparations in the 16 key cities in China was 696 million yuan, up 2% from the previous year.The CFDA has issued 45 registration approvals, 12 of which are raw materials and 22 preparations.Valsartan in the TOP five producers, novartis's generation occupy 87.85%, flat hin occupy 4.25% of southern shandong Bert, CRC secco's guangzhou yue occupied 2.5%, changzhou SiYao valerian, occupy 2.43%, hainan emperor of up to 1.3%.In 2016, valsartan and compound preparations in China were about 4 billion yuan market and became the leader in the treatment market of hypertension in China.
Figure 5. The valsartan series market in the sample hospital of China's key cities from 2008 to 2016
Compound fixed dose ARB is expected to be worth billions
The guidelines stress the use of sequential therapy for hypertension control.Combination therapy is listed as the foundation of blood pressure control, especially for the elderly hypertension for years, a single drug have been unable to make the blood pressure, choose two or more drugs combination therapy has become the preferred solution.
Research shows that fixed dose of compound preparations (FDC) hypertension treatment to bring greater benefits, taking compound preparations antihypertensive treatment success rate of fixed dose was obviously higher than that of free combination, can achieve smooth step-down, 24 hours a day has good compliance.
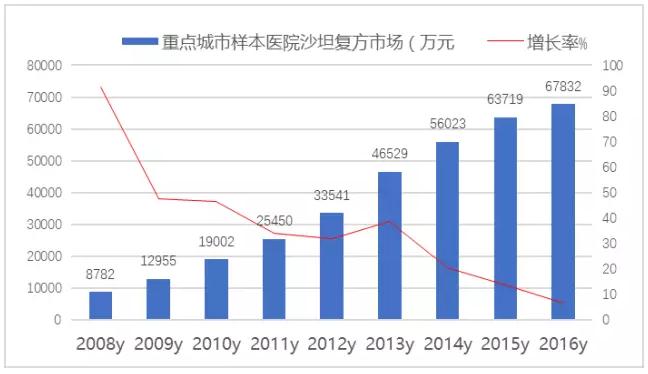
The market for fixed-dose compound ARB products in public hospitals in 16 major cities in 2016 was 678 million yuan, up 6.46 percent from the previous year.The domestic market has exceeded 3 billion yuan and is the leader of the drug market of hypertension.
FIG. 6: shatan compound market, a sample hospital of domestic key cities from 2008 to 2016
At present, the clinical use of compound antihypertensive drugs, fixed dose combination ARB preparations with valsartan amlodipine, hydrochlorothiazide he sand, sand jotham hydrochlorothiazide chloride and valsartan hydrochlorothiazide and telmisartan hydrochlorothiazide, ogilvy sand jotham hydrochlorothiazide and candesartan hydrogen chloride hydrogen thiamethoxam.After compound fixed dose of sand jotham class (ARB) preparation has not yet entered the national health insurance directory, is the bottleneck of its market growth, and entered the 2017 national health insurance directory, on behalf of its entered the high-speed growth area, is expected to reach 10 billion yuan in 2018, the size of the market.
The domestic innovative drug, arishatanl, is expected
, according to data from the CFDA 1.1 class new medicine, sand tank and temple, sand ester, ester approved production in September 2013, the product developed by Shanghai Ellis pharmaceutical technology co., LTD., the domestic patent protection to 2026.Shenzhen litai's letter in October 2012 to spend 340 million yuan for the ester preparation production technology involved with the sand, by jiangsu Ellis (a wholly owned subsidiary Shanghai Ellis) is responsible for production, sand ester apis, letter litai is responsible for the production, the sale of their preparation, sand jotham ester, commodity called xinli temple.The low toxicity of alishatan tablets, the conformance of blood pressure effect is superior to that of similar products, and suitable for long-term use, which is the most promising drug in the market.
In 2016, the sales volume of alishatan, a sample hospital of 16 key cities in China, reached 58.48 million yuan, up 268% from 15.89 million in the previous year, achieving a substantial start.It is a good opportunity to enter the health insurance directory through the drug price negotiation, which will help the manufacturers to implement the expansion plan of the domestic market.
Summary < < <
Currently, sand statins, the original drug covered a major share in the domestic market, domestic brands is still in its infancy, only domestic sand the quality of the drug with the original drug, and the patients, to promote the development of domestic sand jotham drugs.
|
| |
Previous article:Always be check!!!These tablets are the most dangerous
Next article:Medical device operating income and profit, all go bad!I'm to blame?
|
| |
|
|
