| Micro-channel |
 |
|
|

|
|
|
| |
| Consistency evaluation progress data + four provinces waived the list depth profile |
| |
| Author:中國銘鉉 企劃部 Release Time:2017-8-28 10:20:14 Number Browse:753 |
| |
On August 28, 2017, in August 2017, the CFDA published the results of the study on the progress of the evaluation of the quality and efficacy of generic drugs in 289 catalogs.From schedule, 289 directory consistency evaluation target deadline is the end of 2018, consistency evaluation project need cycle at least 15 months, that means not to carry out the evaluation of the project basically can't finish the consistency evaluation.
As shown in table 1, the highest proportion of production enterprises that do not give up evaluation is the exclusive production of enterprise products. As the number of enterprises increases, the willingness of production enterprises to not abandon their products decreases.However, in terms of the proportion of production enterprises that actually carry out the consistency evaluation project, the proportion of products with exclusive products is low, and the proportion of enterprises with two to five enterprises is low.Therefore, the exclusive products are not competitive, and the incentive to conduct a conformance evaluation project is slightly inadequate.
Table 1. Results of the survey of 289 catalogues

(data source: Brenda data V3.2)
The four provinces gave up their approval analysis:
Most drug companies have identified a "bucket list"
Since February 2017, jiangsu, shanxi, zhejiang and anhui were released consistency evaluation enterprise that intends to give up a list of the first published by two provinces jiangsu and shanxi is the technical cooperation development way diplomatically said to give up, on August 2, zhejiang and anhui is released after the give up the list directly.
As shown in table 2, there are 1,500 production batches published by the four provinces, of which 878 production batches belong to the 289 catalogue and 622 production batches belong to non-289 catalogs.
Table 2 gives up the status of the consistency list
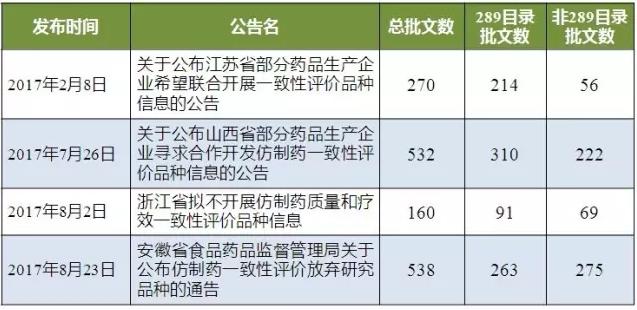
(data source: Brenda data V3.2)
As of August 24, 2017, salty V3.2 data analysis of four provinces to abandon approval number corresponding enterprise from high to low rank, give up the approval of more than 50 companies have Shanxi C&Y Pharmaceutical Group Co., Ltd, wuhu koncz Pharmaceutical Co., Ltd and GaiTianLi Pharmaceutical holdings Group east China Pharmaceutical Co., Ltd. (the huainan good union Pharmaceutical Co., Ltd.), all production enterprises to give up the proportion of approval to the above more than 45%.Therefore, most enterprises in China have preliminarily combed their approval papers and have preliminarily determined the list of products that will carry out the consistency evaluation.
Table 3 the number of enterprises in the top three and the number of their current approvals in the four provinces
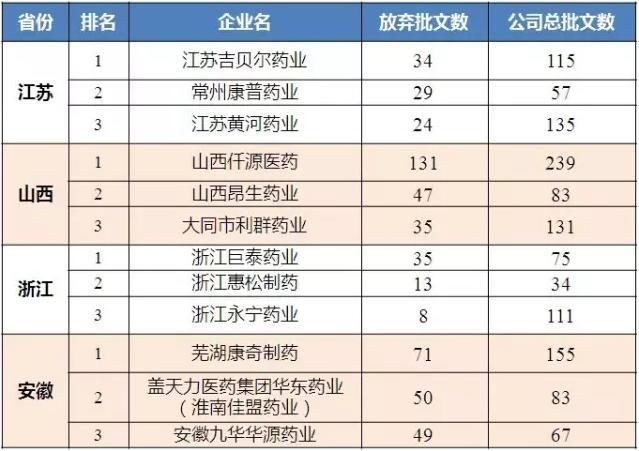
(data source: Brenda data V3.2)
Maximum PK abandonment:
The proportion is directly proportional to the market value of the product
In the 289 catalogue, the products with more enterprises and more enterprises that give up the number of enterprises are many products.From the evaluation formed 100% of the number of product analysis found that 289 directory of the communist party of China 41 product all relevant enterprises are to carry out the consistency evaluation project, the corresponding production companies 33 for 1 ~ 3 products.The higher the proportion of products with more than 5 production enterprises, the higher the market value of the product, the risperidone tablet, gliamurourea and cefuroxime tablets belong to this category.There are 13 products with no production enterprises and 100% of the above products to carry out the conformance evaluation test.
Table 4. Product of 100% consistency evaluation test
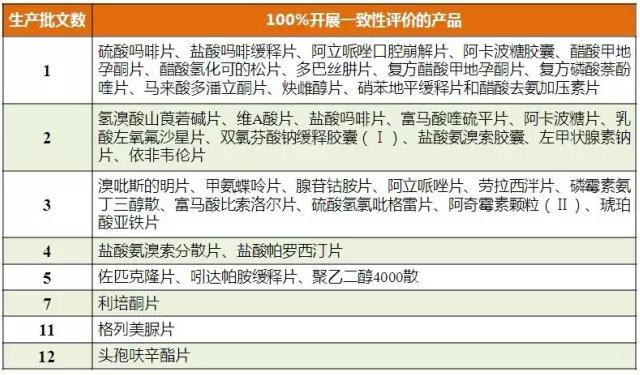
(data source: Brenda data V3.2)
As shown in table 5, jiangsu, shanxi and zhejiang give up approval for top products is 289 catalog of products, and give up approval number number one vitamin C piece of anhui is not 289 catalog of products.The compound sulfamethoxazole tablets and ranitidine hydrochloride tablets were often given more frequency than the number of batches.
Table 5 comparison of the top products in each category
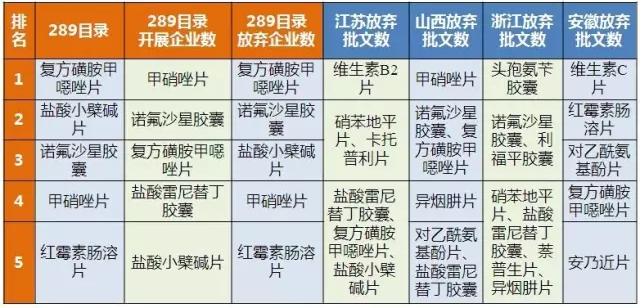
(data source: Brenda data V3.2)
Looking forward to > > >
1. Early warning of shortage of drugs
The more batches of products that are discarded, the more likely they are to be in short supply.There is no national version of the shortage of medicines, and more provinces have issued a shortage in the bidding process, and 38 products have been listed in the provincial list of shortages.In 2015, the proportion of ferrous sulfuric acid ferrous sulfate in the directory of the cheap medicine in jiangxi province was 0% in the 289 catalogue.
Although the national development and reform commission in the operator shortage of drugs and drug ingredients price behavior guidelines (draft) "in the active pharmaceutical ingredients and drug price limits for the shortage, but consistency evaluation will push the product form within the sole and three products, these products is expected to break through the limit price.
2. Release of CRO market?
A total of 3,607 products are in conformity assessment, with the study of pharmacology of 3 million yuan and clinical BE test 5 million yuan estimated, the related r&d investment is expected to cost 28.856 billion yuan.520 base drug is estimated to be worth RMB 350 billion in annual sales volume, but 520 base drugs contain a lot of Chinese traditional Chinese medicine, of which there are 20 TCM products with over 1 billion RMB, corresponding to a scale of about 59 billion yuan.The market size of the 289 catalogue is below 300 billion yuan, and the investment amount of 28856 billion yuan is basically equal to the gross profit of the whole year.
However, the research and development of enterprises is not only the consistency evaluation, but also the process change, injection reevaluation, etc.Therefore, from the perspective of input and output, the research and development of the group will be a big trend, and CRO may not be able to do business of 28856 billion yuan.
|
| |
Previous article:The ministry of industry and information technology announced that in the first half of 2017, the most profitable part of the film is the beverage.
Next article:Eleven pharmaceutical biotechnology companies doubled their gross profit margins by more than 80 percent
|
| |
|
|
