| Micro-channel |
 |
|
|

|
|
|
| |
| The proton pump inhibitor is the market leader in the market |
| |
| Author:中國銘鉉 企劃部 Release Time:2017-9-6 16:00:34 Number Browse:1540 |
| |
Medical network on September 6 - editor said: according to the world health organization, according to data from the global incidence of digestive system was 10% ~ 12%, and 11.2% incidence of digestive system of our country town, and in many developed countries in Europe and America were little changed. Digestive diseases are most common in canker, superficial gastritis and chronic atrophy gastritis. Gastric ulcers and duodenal ulcers are called peptic ulcers. Its pathogenesis is that the invasion effect of gastric acid and pepsin is out of balance with the defense ability of the mucosa, and gastric acid can produce self-digestion of the mucosa. If the comparison of membrane barrier to a "roof", gastric acid and pepsin as "acid rain", "roof" leakage met not too strong "acid rain" or "acid rain" corrupt the normal "roof" are likely to affect the occurrence of peptic ulcer.
Currently, Proton pump inhibitors (proton-pump inhibitors) PPI) is the most advanced to a category of drugs in treatment of peptic ulcer, it through efficient rapid inhibition of gastric acid secretion and removal of h. pylori and achieve rapid healing of ulcer, is more than ten years clinical correlation disease is the most widely used in the treatment of acid, curative effect is the best medicine.
Proton pump inhibitors are market darlings
The first proton pump inhibitor, omeprazole, was developed by astra in 1988. Following omeprazole, the world has developed a series of proton pump inhibitors such as lansolazole, pantoprazole, esomeprazole, and leibelazole. The overseas proton pump inhibitor reached its highest peak in 2007. Under the imitation competition, the global market gradually entered the downhill stage, while the market of proton pump inhibitor in China was still in high speed.
Catalogue of 2017, national health care medicines included in the treatment of peptic ulcer and gastric esophagus reflux prescription drugs have 12, proton pump inhibitors are the largest number of the classes, respectively: first generation PPI drug omeprazole, panxi tora azole and LAN sola thiazole; The second generation of PPI drugs, esomeprazole, eprazole, and leibelazole. The new generation of PPI drugs has the characteristics of quick effect, long duration and small side effects, which is the main reason for the rapid growth of the market.
Network according to the m key cities public hospital chemical medicine, according to data from the terminal competition in 2016, domestic 16 key cities public hospital proton pump inhibitor medication amount reached 4.5 billion yuan, an increase of 6% over the previous year, present a rising trend.
Proton pump inhibitor is stronger than H2 receptor blocker and is a popular market in recent years. China has approved the listing of the proton pump generics of many pharmaceutical companies, improved the product structure and increased market competition. Newly approved drug makers have challenged manufacturers for years to come with new products, new processes and new forms of preparation.
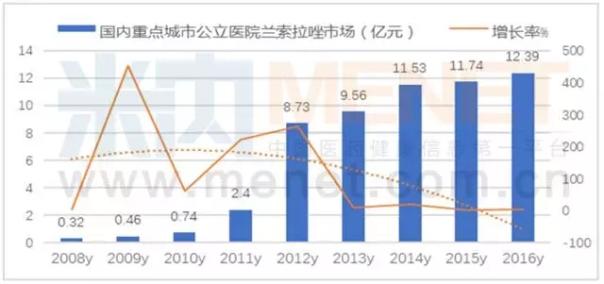
Lancollazole led the PPI market
Network according to the m key cities public hospital chemical medicine terminal competition pattern, according to data from 2016 domestic 16 key cities public hospital market ranks first in the PPI is orchid sola, its sales of 1.239 billion yuan, increased by 5.52% year-on-year, for key cities public hospital top 10 ranks, 8.34% of the share.
It was originally developed by takeda, Japan, and the product is called dacron. In 2008, the development of an injection of lansorazole powder in the pharmaceutical industry of the jiangsu province was gradually transforming the lancazole market. So far, the CFDA has approved 70 pharmaceutical companies to produce the lansolazole production approval.
The antibacterial activity of the helicobacter pylori is four times higher than that of omeprazole. Its bioavailability is higher than the same species, with high incidence of adverse reactions and strong lipid. There is an obvious therapeutic effect on the ulcer disease of chronic ulcer and receptor antagonist.
In 2016, the domestically-produced lansolazole used in public hospitals in China's major cities occupied 96 percent of the market, and the original import drug, dacron, accounted for 4 percent of the market. Of the top 5 sales figures, jiangsu osikang accounted for 30.60 percent, shandong luoxin of 25.52 percent, Beijing yue kang 13.46 percent, changzhou 4 medicine accounting for 10.28 percent and takeda company 4.01 percent. According to the data of the competition pattern of chemical drug terminal in public hospitals in the key cities of mienet, the powder injection agent accounted for 89.62%, and the capsules accounted for 5.44% and the tablets accounted for 4.94%.
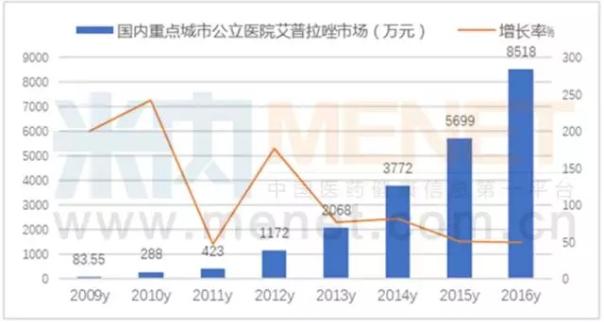
Prazole, like a tiger
Ai oprah, commodity called "one lian", is the first and only our digestion treatment field of innovative drugs, 2017 new drugs into the catalog of national health care drugs, listed by the group research method. On December 11, 2007, prazole was awarded the new drug certificate and its production approval issued by the state administration of food and drug administration, and was first listed in China in 2008; 37 patents were granted for invention, and 23 patents were granted in the United States and Europe.
Apprazole is an irreversible proton pump inhibitor of phenylproimidazole, which is selectively entered into the gastric wall cells after oral administration and is transformed into a subsulfonamide active metabolite to play a therapeutic role. It has the advantages of significantly improving similar drug suppression time, individual difference and drug interaction. And its use in manufacturing API key technology of oxidation and crystallization and orientation drug release technology, to overcome the problems in the process of research and development and industrialization, won the 2015 national scientific and technological progress second prize.
According to the competition pattern of chemical drug terminal in public hospitals in key cities of mienet, the sales of prazole in public hospitals in 16 key cities in 2016 were 85.18 million yuan, a year-on-year increase of 49.47 percent. In the first half of 2017 domestic ai oprah azole sales of 209 million yuan, increased by 46.77%, with more than 20 domestic provinces, municipalities and autonomous regions of new medicare catalog, in 2017, ai oprah azole breakthrough 500 million sales mark have a shoo-in.
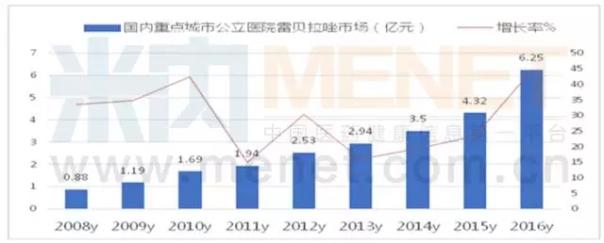
Rabeprazole growth was 44.53%
Leibelazole is a second generation of new proton pump inhibitors. On August 19, 1999, the FDA approved eisai ray Bella azole listed company, called Pariet goods, it is suitable for the treatment of erosive gastroesophageal reflux disease, and after the maintenance treatment, and is suitable for the treatment of duodenal ulcer and assist Mr Ellison syndrome.
Rabeprazole azole resistance is a kind of secretion of reversible proton pump inhibitors, has high value of PKaA, its anti secretion activity in vitro, 2 ~ 10 times stronger than omeprazole, oral can rapid activation in the body, play a role of acid suppression to combine with proton pump. Remberazole entered the Chinese market in February 2000, with a product named polly.
According to the competitive pattern of chemical drug terminal in public hospitals in the key cities of mienet, public hospital of public hospital in 2016 was 625 million yuan, up 44.53% year on year. The TOP5 manufacturer's market share is respectively is eisai (China) pollitt enteric-coated metformin hydrochloride 21.6%, Jiang Suji sichuan's economy enteric capsules 18.51%, nanjing long Australia 17.01% of SJM flat powder injection, chengdu dickon Ann, at least 10.81% of enteric-coated metformin hydrochloride in jiangsu rui potter of enteric-coated metformin hydrochloride was 10.66%.
Be worth what carry is, from the thunder Bella azole agents registration approval and the analysis of the competitive landscape, rabeprazole TOP5 azole market makers formulations for a more reasonable proportion, the catalog of national health insurance in 2017 first choice under the situation of oral preparation, rabeprazole azole will become the next two years one of the varieties of considerable market prospect.
|
| |
Previous article:5. Overview of the semi-annual report of a-share listed pharmaceutical manufacturing enterprises in 2017
Next article:Shaanxi county, town, village, also need to implement consumables "two ticket system"!
|
| |
|
|
