| Micro-channel |
 |
|
|

|
|
|
| |
| Consistency evaluation is on the ground: non-289 catalog 30 products are expected to be LOGO |
| |
| Author:中國銘鉉 企劃部 Release Time:2017-9-7 10:42:10 Number Browse:974 |
| |
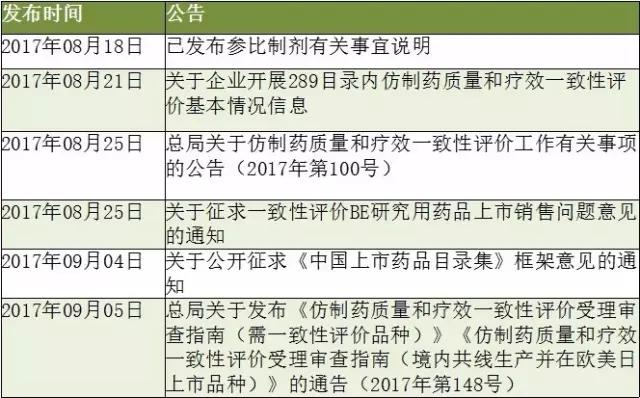
Medical network - on September 7, in August 2017, the CFDA/CDE for consistency evaluation continuously sent several big announcement (as shown in table 1), the announcement included within 289 directory for the current progress of generic drugs and announced "through consistency evaluation" logo figure, generics Orange Book of China (" China listed drugs directory set ") prototype also released a draft, this means that the consistency evaluation started to enter the policy execution.
Table 1: CFDA/CDE's recent policy overview
(data source: Brenda data V3.2)
289 catalogue variety: reference preparation is not yet fully prepared
Consistency according to the administration about the quality of generic drugs and curative effect evaluation of announcement of relevant matters, to facilitate enterprises to select reference preparations, the CFDA administration will take on the implementation of the general office of the state council about to carry out the quality of generic drugs and curative effect evaluation opinion consistency > announcement of relevant matters of no. 106 (2016) attached to 289 varieties of the original enterprise drug list and publish to the society, as a reference for enterprises to choose reference preparations. As of September 6, 2017, the CFDA announced the release of the eight batches of reference preparations, and 112 of the 289 catalogue products were still unpublished.
In addition, CFDA has committed to publicize the total number of reference preparations filed by the general administration. In contrast, companies are relatively active in applying for reference. On September 4, 2017, China's food and drug verification research institutes publish enterprise archival filing reference preparations (May 20, 2016 to August 20, 2017) data show, 289 directory products in 46 no enterprise declare reference preparations for the record.
Companies and countries have not disclosed 23 products. However and announced by the CFDA enterprises to carry out the quality of generic drugs within 289 directory and curative effect evaluation of the basic situation of consistency is found that not only five products evaluation, consistency is carrying out the rest of the products have enterprise. This means that there is still information difference between the company's record reference and the actual consistency evaluation.
Table 2: there is no list of companies and countries on the preparation list

(data source: Brenda data V3.2)
289 varieties: 30 drugs are identified with "consistency"
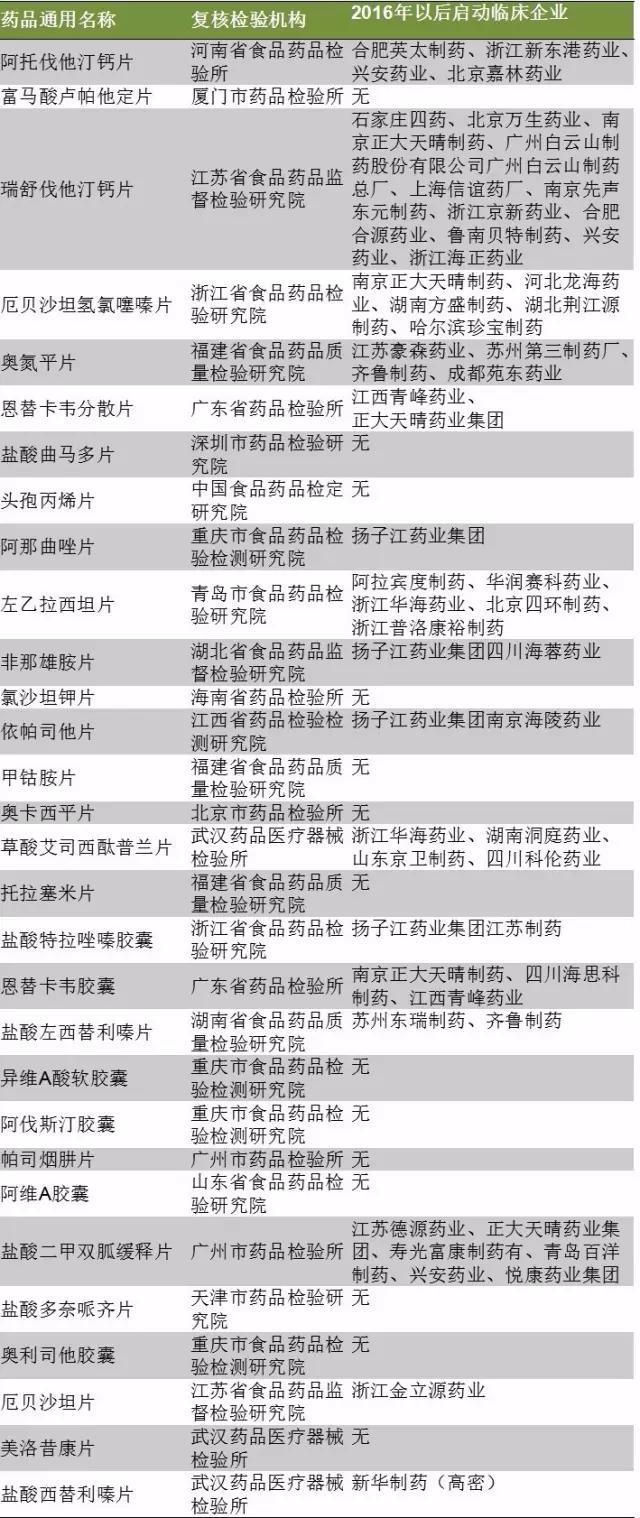
Since June 2017, the evaluation office of the quality and efficacy of generic drugs has released a list of two batches of 289 categories of review and verification institutions, including 30 products.
The author believes that the 30 products should be declared in conformity assessment, and the quality and effectiveness of the generic drug will be released by the office.
As shown in table 3, fumaric acid Lou palmer he slice, tramadol hydrochloride, cefazolin propylene, potassium chloride sand pills, A cobalt amine, oqa xiping pills, tora crunching of cornflakes, different vitamin A weakness in the capsule, avastin capsule, individualized, enjoying A capsule, donepezil HCL, orlistat capsules and los ever since 2017, "temporary undeclared clinical.
Shiyao group of tramadol hydrochloride and donepezil HCL, huahai pharmaceutical potassium chloride sand jotham, the beauty of huarun double crane yesterday "have the ANDA, in addition to shiyao donepezil HCL tablets are domestic product already on the market. It is estimated that 14 of the products that have not been launched clinically but have entered the review list in 2016 will be consistent with the production of domestic collinear and listed on Europe and the United States.
After 2016 start clinical 16 products, Yangtze river pharmaceutical group co., LTD., the maximum number of exclusive launch clinical products, a total of three, respectively is anastrozole, finasteride pills and in accordance with Mr He. In terms of products, rishuvastatin calcium tablet has the highest clinical heat.
Table 3: the clinical declaration of 2016 to date of the variety of the compound inspection institution 289
(data source: Brenda data V3.2)
Think about the < < <
At present, the main policy of conformance evaluation is: when the drug is centralized, the manufacturer enjoys the right of preferential price. However, the generic quality and efficacy consistency evaluation office has not published a list of varieties that have been evaluated by consistency. This also means that "with varieties of drugs through consistency evaluation of manufacturing enterprises to achieve more than three, in such aspects as drug centralized purchasing no longer choose not through consistency evaluation of variety" and "through consistency evaluation of drug varieties, social security will give appropriate support, in terms of payment in clinical medical institutions should be prior purchasing and preferable" policy of actual execution.
It is worth noting that because of the reform of the "zero addition" of medical institutions, the hospital began to host the pharmacy, and the market for oral medicine was gradually transferred from the medical institution to the pharmacy outside the hospital. However, pharmacies are not subject to the policy of centralized procurement of drugs, which means that pharmacies can still sell varieties that fail to be evaluated in conformity. Therefore, from now on, the policy of the variety of the conformity assessment is limited, can only hope that the medical insurance payment in the context of the use of medical institutions.
|
| |
Previous article:Ningxia's "two-ticket" policy has a new change province, not a single vote!
Next article:The increase in the price of bio-similar drugs may exceed 15%
|
| |
|
|
