On September 15, the medical website entered September, and the traditional Chinese medicine injection life began to suffer!
Be restricted to
In February 2017, the people club department released a new version of the national basic medical insurance, inductrial injury insurance and maternity insurance drug catalogue (2017 edition) "(hereinafter referred to as the catalog of medicines), among them, there are varieties of traditional Chinese medicine injection in 49, however, the" catalogue of drugs for traditional Chinese medicine injection using the strictly limited, limited varieties reached 39, in limited varieties, including ShuangHuangLian injection, qing ling injection, ShuXieTong injection varieties such as the limit of more than secondary use, medical institutions and severe, the limitation of diseases, the constraints mean basic-level hospitals if use will not be reimbursement.
Starting on September 1, 2017's new catalogue of medicines is being implemented in several provinces, which means TCM injections will be subject to multiple restrictions.
Similarly, a few days ago, a social security bureau issued in towns and townships of the catalog of limit level 2 above hospitals drug varieties (hereinafter referred to as the catalog) are circulating in the industry, the catalog shows: from September 1, package Gua thrombosis blood plug,, open spirit, ShuangHuangLian and salvia miltiorrhiza 27 kinds of commonly used traditional Chinese medicine injection at the grassroots level used in towns and townships shall not submit an expense account, it is understood that the "directory" is the source of henan province.
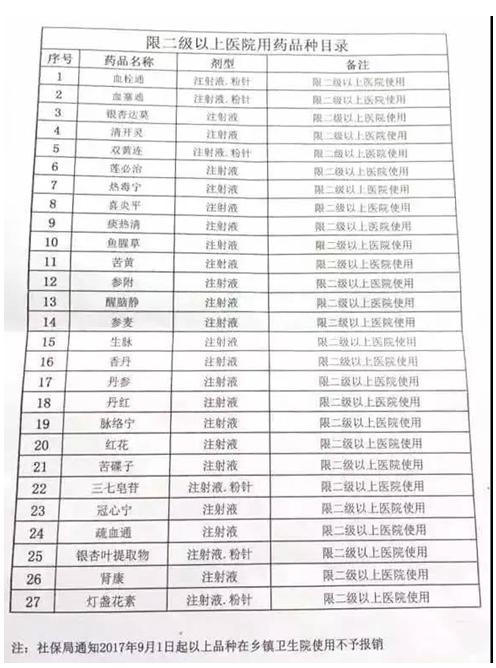
Have industry analysis, the list or not only applicable to henan province, put it in, that is, from September began, village clinics, community health center, in towns and townships under secondary medical institutions will no longer use more than 20 kinds of traditional Chinese medicine injections, unless at his own expense, in addition, the drugs limited after purchase is or will be a problem.
Several listed companies will be affected
A senior industry source pointed out that, after the cultivation of 2009 and 2012, the Chinese medicine injection of Chinese medicine became fast variety and large variety. The adjustment of the health care catalogue, as well as the fees charged by the provinces, will make it harder for traditional Chinese medicine injections, which will have the biggest impact on TCM injections in the national basic drug catalog.
Therefore, several listed companies, including zhongheng group, kunming pharmaceutical group and yibai pharmaceutical, have also issued announcements on the subject of their own restricted use of injection products.
Zhongheng group released on the subsidiary products for injection via blood clots (lyophilization) limited medical insurance reimbursement at the grass-roots level influence complement announcement said the vial thrombosis (lyophilization) belong to the company's flagship product, the product in the 2016 annual sales income 1.383 billion yuan, 82.81% of the company's business revenues, secondary medical institutions under the sales accounted for 30%; In the first half of 2017, the sales revenue of the injection of thrombong (freeze-dried) was 805 million yuan, accounting for 86.19% of the company's operating revenue, and 29.8% of the sales of medical institutions under secondary level.

Announcement, with many provinces have started to implement the new medicare catalog, access (lyophilization) injection with thrombus in henan and other provinces and regions sales effect still need further evaluation, taking henan province as an example, the injection with thrombus tong (lyophilization) in the province in 2016 the province's sales of 080 million yuan, at the grass-roots level accounted for about 30%, after the policy implementation, is expected to affect the proportion of 5% 15%.
For limited impact, zhongheng group, said the company is actively respond, will continue through the strict controls of product quality, strengthen the promotion, strengthening marketing management, fine management, strengthen the research and development efforts, and increase research and development personnel and marketing team, to do a good job in market development, the authors advocate efficiency to ensure the company's products in the market dominant position in the field of the core.
Kunming group released on blood plug injection products medical insurance directory in the new explanation of the announcement said, according to the product flow to the data statistics, 2016 to 2017 in the first half of the company's blood plug tong injection (injection booster injection) in the secondary hospital (excluding secondary hospital, hereinafter referred to as the "grassroots hospital") sales accounted for about 45-50%, in 2016, blood plug industrial sales income of 660 million yuan, under the secondary hospital sales accounted for 47%, in the first half of 2017 the sales of 40 million yuan, the grass-roots hospitals accounted for 46%. As the new national health insurance directory in various provinces and cities have fall to the ground, blood plug tong injection (injection booster injection) in various provinces and cities basic-level hospital medical insurance payment will be limited when using, the sales will be affected by a certain part of the hospital market.

Kunming group said, assuming that the national market in accordance with the September 1, 2017 began to implement the new national health insurance directory, two varieties are close to the company 2017 annual industry sales revenue by around 10-15%, annual net profit of about 5-10%.
For this effect, kunming group said, products that are the new medical insurance directory limit state level hospitals use, but not limit according to the indications for use, the company will in the other competing products limited indications in the market, and clinical research, academic promotion activities, increase the limited market sales.
Yi hundred pharmaceutical announcement said, compared with 2009 health insurance directory, the company's ginkgo da mo injection in the new medical insurance directory restricted by increasing indications, specifically for medical institutions "limit level 2 or more and have a clear ischemic disease of heart head blood-vessel acute stage patients". In 2016, the sales revenue of ginkgo daemo injection reached 420 million yuan, accounting for 11.44% of operating income and 13.10% of gross profit. In the first half of 2017, sales revenue amounted to about 200 million yuan, accounting for 9.95 percent of revenue, 12.33 percent year-on-year growth and 11.69 percent in gross profit. The sales terminal structure of the product is about 20% of the secondary (non-secondary) hospital market. It is understood that in this market, yibai pharmaceutical has the largest market share.

"There will definitely be losses, but there has been no real loss," said li gang, head of the company. The introduction of new health care from September 1st, reflected in the doctors' habit of medicine, is also a process, so the forecast will not have much impact on sales this year.
Why is the limit of
The safety and effectiveness of traditional Chinese medicine injection has been mentioned repeatedly, which is also the "biggest stumbling block" to its development.
2015 national adverse drug reaction monitoring, according to the annual report 2015 national adverse drug reaction monitoring network received TCM injections reported 127000 cases, including serious report 9798 cases (7.7%), Chinese medicine adverse reactions/events in 2015 report, the injection ratio is 51.3%.
In 2016, the annual report of the national drug adverse reaction monitoring showed that the proportion of injections in the 2016 Chinese medicine adverse reaction/incident report was up to 53.7 percent. Report also pointed out that from the involved dosage formulations and traditional Chinese medicine (TCM) injections is higher, need to continue to focus on its security risk, look from the drug category, is primarily concerned with promoting blood circulation to remove blood stasis, heat-clearing and detoxicating, nourishing Yin, cool open TCM injections, prompt should continue to pay attention to the above categories and drug risk, risk control measures in a timely manner.
It is known that traditional Chinese medicine injection adverse reactions mainly involves systemic damage, respiratory system, skin and its accessories damage, etc., including allergic reaction, anaphylactic shock, chills, fever, difficulty breathing, chest tightness, heart palpitations, itching, rash, nausea, vomiting, etc.
In June the 12th session of the National People's Congress on the 28th session of the standing committee, the state food and drug supervision and administration bureau chief jingquan in "state council on drug management work report" has pointed out that in the drug quality and safety risks. The injection of injection, especially early approval of the listed Chinese medicine, is weak in the basic research of safety and effectiveness. Some production enterprises cut corners, use fake raw materials, change the production process without authorization, and seriously affect the safety and effectiveness of drugs. For this, the next step will be to promote the effectiveness and safety evaluation of listed injections in stages.
In fact, as early as eight years ago, China launched a round of traditional Chinese medicine injection reevaluation work. On July 16, 2009, the state food and drug administration issued "about to do a good job of TCM injections safety evaluation again notice and a series of documents, full swing TCM injections evaluation again, but industry observers point out that until now, the evaluation work doesn't really be born again. The reason is that there are medical personage points out, on the one hand is the drug firms do not like to TCM injections and evaluation work, on the other hand is to evaluation the top design is not clearly related conditions.
However, according to the industry, in 2018, before and after the country is likely to be proprietary Chinese medicine carried out a similar "curative effect, safety and risk evaluation to" true, and in the form of "listed again after evaluation", when proprietary Chinese medicine enterprises will usher in a big reshuffle, while TCM injections as the biggest in the proprietary Chinese medicine prescription drug dosage forms, affected by the impact will be inevitable.
|
