2016 CFDA approved 45 new medical devices
Source: China's inscription Xuan planning department release date: 2016-12-9 9:24:23 views: 2
Medical network on December 9 - following the November 15, the CFDA medical equipment technology evaluation center (hereinafter referred to as the "CMDE) published include hangzhou commitment medical treatment of sacral nerve stimulation system for a total of seven innovative medical devices to enter the special program, on November 22, CMDE again issued 2016 11th innovation medical instrument special approval to apply for review the results of the public, to agree to one application program to the innovation special approval process.
In this paper, the inventory by the end of this year on December 7, 2016, through the innovation of medical equipment in 2016 45 product special approval to apply for review; There are 2 companies (superscript) marked on each have 3 item, about the project of in vitro diagnostic (background for the green) a total of eight.
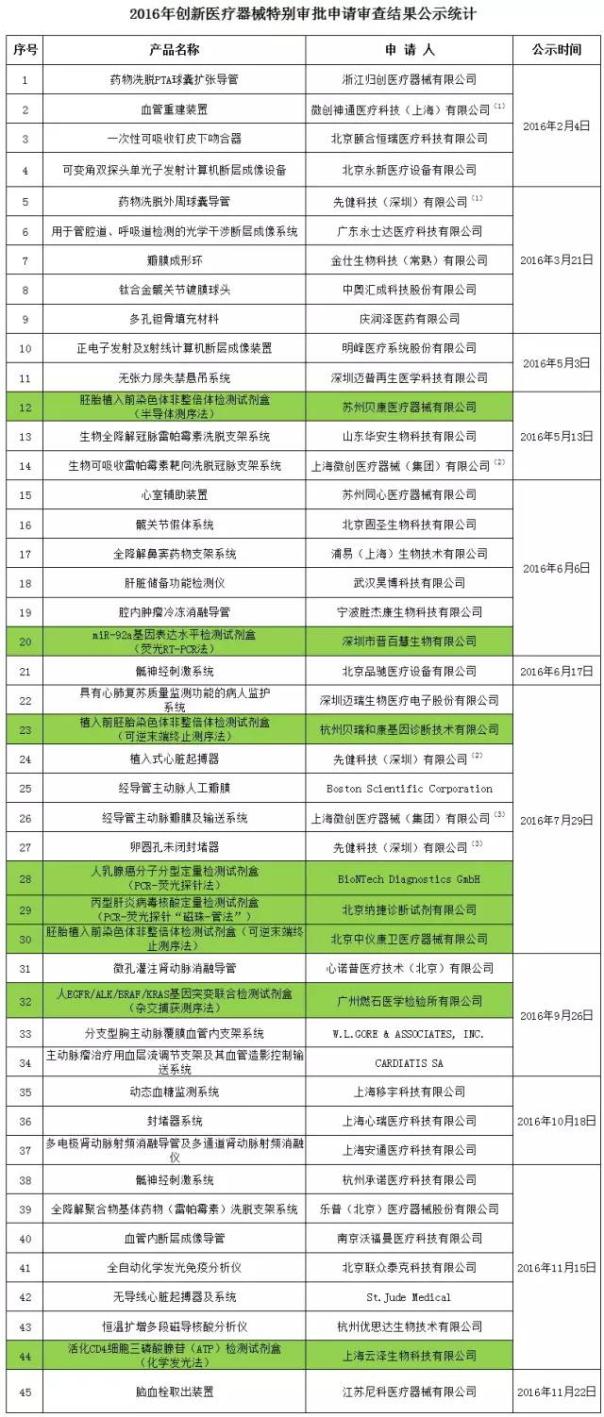
Note: minimally invasive avatar is a wholly owned subsidiary of Shanghai minimally invasive.
Attachment: nearly two years into the approved "innovation medical instrument special approval procedures" products
In 2015, a total of 29 item into the CFDA innovation medical instrument special approval to apply for review, including Shanghai minimally invasive 2 item, about the project of in vitro diagnostic (background for the green) a total of five.
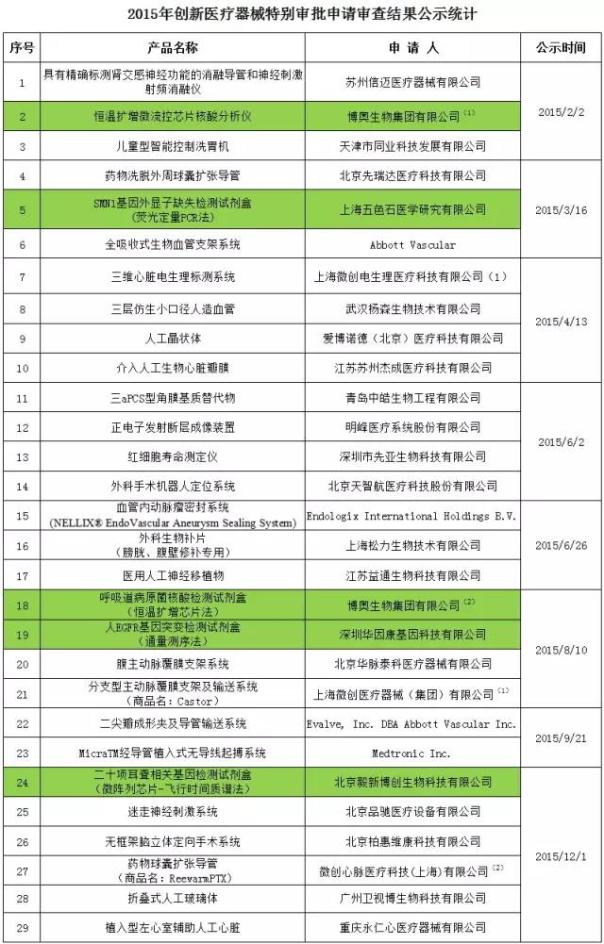
Note: minimally invasive heart medical technology, a wholly owned subsidiary of Shanghai minimally invasive.
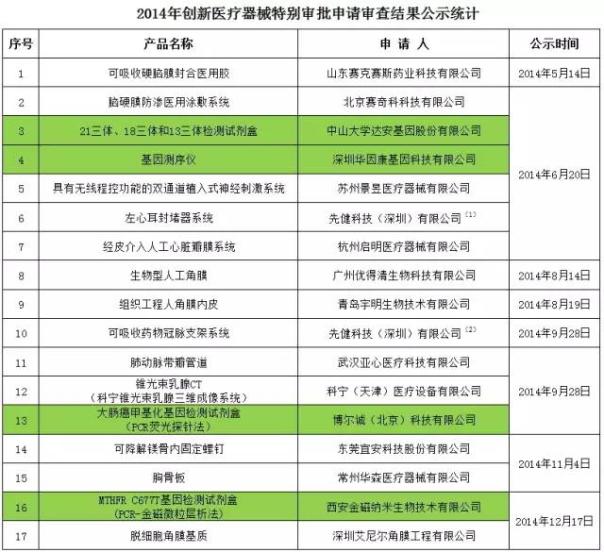
In 2014, a total of 17 products enter the CFDA innovation medical instrument special approval to apply for review, the first national health technology (shenzhen) co., LTD. Have 2 item, about the project of in vitro diagnostic (background for the green) a total of four.
|
