Medical network on December 16 - to enter at the end of the year, guangdong food and drug administration intensive medical instrument flight inspection work.
Less than a month, has notified the two batch for the medical equipment production and the quality control standard for the implementation of the flight check the situation of production enterprises, with a total of two machinery companies were ordered to stop production rectification, eight interview, 11 rectification within a time limit.
Since November 7, the guangdong food and drug administration also organized the 2016 annual medical equipment management enterprise flight check, more than 12 machinery makers have been ordered to stop business operations for rectification, 58 rectification within a time limit.
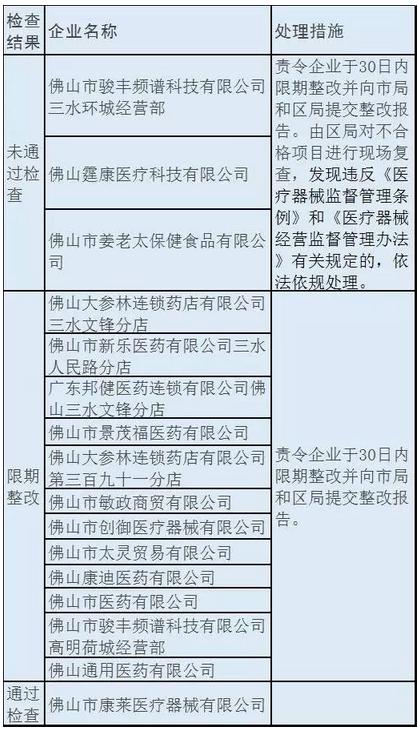
In addition, from the actual flight inspection of food and drug administration. Foshan city food and drug administration in November 8 to 9, organize the implementation of the medical equipment management double random flight check, the results of 16 by a flying object, only 1 through examination, 12 were ordered to make correction, and three failed the examination, in addition to make correction, will also accept on-site review and other processing.
Bureau of guangdong province in 2016 the second batch of machinery companies flying test work: four questioning, six rectification
Release date: 2016-12-14
Flight test purpose: "medical equipment production and the quality control standard for implementation
Flying test object: the production of artificial lens, in vitro diagnostic reagent, anesthesia machine, disposable use asepsis self-destruction syringe needle, digital color ultrasonic diagnostic system, blood purification equipment products such as medical device manufacturing enterprise.
Check the key: raw material procurement control and validation, quality control, production management, etc.
Results: found that quality management system defects in general more, questioning 4 companies; Defects commonly found quality management system, the rectification within a time limit of 6 companies.
Enterprise main problem: checked products for customer requirements change, not to change to the product safety, efficacy to evaluate the risk of; Production record is not clear, complete, if not record production process parameters and main equipment serial number, etc.; Raw materials, the product in the process of production without state identification; Process water storage tanks and pipelines by file not require regular cleaning and disinfection.
Bureau of guangdong province in 2016 the first batch of machinery companies fly: two production, four interview, five rectification
Release date: 2016-11-23
Flight test purpose: "medical equipment production and the quality control standard for implementation
Check key: raw material procurement control and validation, the source of the material is legal, if the traceability, whether with qualified certificate; Incoming goods inspection, process inspection and final inspection records are true and complete; Production management, enterprise whether to adopt effective means to realize from the storage of raw materials, production process, the factory until the whole sales process clear traceability; Production operating rules on the design and implementation; Production records are true, integrity.
Results: found that quality management system, there are serious defects shall be ordered to shut down 2 rectification enterprises; Defects commonly found quality management system more, questioning 4 enterprises; Defects commonly found quality management system, rectification within a time limit five enterprises.
Bureau of guangdong province in 2016 the second batch of machinery comac inspection: six out of business, 22 rectification
Release date: 2016-12-15
Flying test purpose: the medical equipment management and the quality control standard for on-site inspection guidelines "and" medical equipment cold chain (transportation, storage) management guide, etc
Flight test object: engaged in cold chain management of medical equipment management enterprise
Check key: enterprise and the legal qualification of the product sales, enterprise quality management personnel on-the-job on-the-job sulk, cold-chain management system implementation, upstream and downstream channel are true, legitimate, the record is complete, etc.
Results: most of the business enterprise compliance; But still there are some companies do not meet the requirements of medical equipment management and the quality control standard, etc., six were asked to stop business operations for rectification, 22 were ordered to make correction.
Add: list to shutter its operations for enterprises
1. Shenzhen minister a technology co., LTD
2. Shenzhen love d di medical technology co., LTD
3. Shenzhen rui kang technology co., LTD
4. Shenzhen billiton sichuan investment co., LTD
5. Shenzhen jia Ming reagent co., LTD
6. Yunfu NingHua medical instrument co., LTD
The rectification within a time limit companies list
1. Shenzhen lam pharmaceutical co., LTD
2. Guangdong boat xiang trade co., LTD
3. Dressing to huizhou electronics co., LTD
4. Huizhou hong already pharmaceutical co., LTD
5. Fly huizhou medical technology co., LTD
6. Shanwei cities ding cheng biological technology co., LTD
7. Dongguan east medical instrument co., LTD
8. Guangdong dongguan national medicine group co., LTD
9. Dongguan taian pharmaceutical co., LTD
10. Yangjiang feng xin trading co., LTD
11. Yangjiang Jiang Rui medical equipment co., LTD
12. Yangjiang HuaCheng medical instrument co., LTD
13. Yangjiang embellish of pharmaceutical chain co., LTD
14. Zhanjiang bo trade co., LTD
15. Zhanjiang anzheng pharmaceutical co., LTD
16. Spirit of zhanjiang ocean medical equipment co., LTD
17. Maoming colorful days medical instrument co., LTD
18. Maoming Garrett medical instrument co., LTD
19. Maoming city health state medical instrument co., LTD
20. Maoming immortality pharmaceutical co., LTD
21. Yunfu city building macro pharmaceutical co., LTD
22. Yunfu city kangda pharmaceutical co., LTD
Bureau of guangdong province 2016 year first machinery comac inspection: six out of business, 36 rectification
Release date: 2016-12-5
Flight test purpose: administration of medical devices on the regulation of circulation management behavior in the field of announcement no. 112 (2016), etc
Flight test object: engaged in cold chain management of medical equipment management enterprise in the province
Check key: the enterprise the management main body, the product and personnel qualification meets the requirements, strict quality management system is established and effective implementation, cold chain storage and transportation conditions whether complete, marketing channel is complete specification, all the records are in, etc.
Results: most of the enterprise to operating according to regulation in accordance with the law; For key projects do not conform to the requirements or general projects do not conform to the requirements of the number > 10% of the enterprises, ordered to stop business operations for rectification six; On general project does not meet the requirements of the number of 10% or less, shall be ordered to make rectification within a time limit of 36.
Was found problems in the enterprise: quality management system implementation is lax, warehouse and equipment management is not complete, sales records, cold-chain management does not reach the designated position, etc.
Add: list to shutter its operations for enterprises
1. Guangzhou hermann medical equipment co., LTD
2. Guangzhou Kang Cheng medical instrument co., LTD
3. Foshan di Wallace trade co., LTD
4. Zhongshan zhongjing medical instrument co., LTD
5. Jiangmen Mr Medical instrument co., LTD
6. Chaozhou day cheng pharmaceutical co., LTD
The rectification within a time limit companies list
1. Guangzhou health medical technology co., LTD
2. Guangdong jinpeng medical instrument co., LTD
3. Guangzhou guangdong au biological technology co., LTD
4. Guangzhou is steel trade co., LTD
5. Guangzhou clinical medical instrument co., LTD
6. Zhuhai lian Po pharmaceutical co., LTD
7. Zhuhai zhongshan d contour pharmaceutical co., LTD
8. Zhuhai cumming pharmaceutical co., LTD
9. Zhuhai Deere biological engineering co., LTD
10. The shantou special economic zone longhu half a continent reagent co., LTD
11. Shantou times, medical equipment technology co., LTD
12. Guangdong antell pharmacy co., LTD
13. Shantou shun kang medical instrument co., LTD
14. Rhea, foshan medical product development co., LTD
15. Foshan city kangpu pharmaceutical co., LTD
16. Guangdong hui morning medical technology co., LTD
17. Shaoguan city kang medical instrument co., LTD
18. Meizhou city hui long medical instrument co., LTD
19. Meizhou city raw pharmaceutical co., LTD
20. Meizhou city jin hong biotechnology co., LTD
21. Zhongshan biological engineering co., LTD
22. Guangdong Mr Kony medical instrument co., LTD
23. Guangdong medicine jiyuan hall pharmaceutical co., LTD
24. Jiangmen mercy medical instrument co., LTD
25. Zhaoqing city kang medical instrument co., LTD
26. Zhaoqing city hua tuo trade development co., LTD
27. Guangdong hua yu pharmaceutical co., LTD
28. Guangdong di optimal trade co., LTD
29. Qingyuan city homologous medical instrument co., LTD
30. Chaozhou xiang jin pharmaceutical co., LTD
31. Guangdong cape biotechnology co., LTD
32. Guangdong qi China medical equipment co., LTD
The age of 33. Guangdong pine pharmaceutical co., LTD
34. Guangdong ring, health medical equipment co., LTD
35. Guangdong Kang Baoyuan pharmaceutical co., LTD
36. Guangdong shunde neutral pharmaceutical co., LTD
Foshan machinery comac inspection: 12 rectification within a time limit, the three failed the examination
Time: 2016.11.8-11.9
Results:
|
