Medical network - on January 6, according to statistics, in 2017, the compound patent in China is on the verge of failure of an NCE (new molecular entities) nearly 20 new drugs, including the global annual sales of more than $1 billion blockbuster products have three, respectively, of its drug lenalidomide, Gilead tenofovir, BMS, the wei.
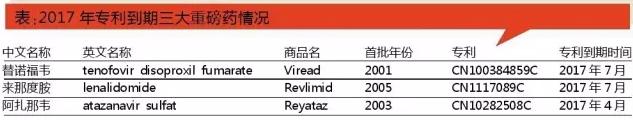
In 2015, the three kinds of branded global sales of more than $8.048 billion, are mainly distributed in the treatment of anti-tumor and anti-infection. Among them, its drug lenalidomide sales as high as $5.801 billion in 2015; The BMS, that sales were $1.139 billion; Gilead tenofovir sales of $1.108 billion.
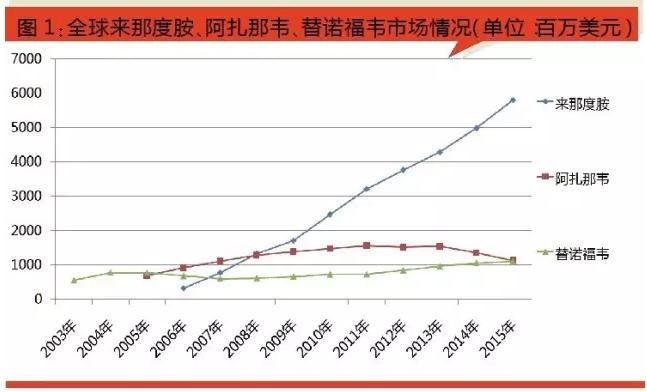
According to the data sample of hospital in China, in the third quarter of 2016 years ago, the drug lenalidomide sales of 10.42 million yuan, tenofovir sales of 80.02 million yuan. Of tenofovir for the 2016 domestic medical market growth is more outstanding products, mainly with glaxo tenofovir slashed prices in 2016, is expected to tenofovir sales samples of hospital in China is expected to more than one hundred million yuan.
Switzerland after the
In the Chinese compound patents expire time in July 2017
Global: sales is growing rapidly
Drug lenalidomide its development, by the United States commodity called "Revlimid. The drug by the FDA in 2003 as a rare diseases drugs and into the fast channel of examination and approval, 2005 approved for the treatment of myelodysplastic syndrome (MDS), and then in 2008 approved for the treatment of multiple myeloma (MM). In addition, the drug lenalidomide also has therapeutic effect for a variety of leukemia and solid tumor, in June 2013, approved by FDA drug lenalidomide treatment cell lymphoma new indications. Drug lenalidomide indications continues to expand, is expected to expand the market space.
According to the world's bestselling drug data statistics, in 2015 years that degree of amine sales as much as $5.8 billion, a 16.5% increase from the same period. From the point of sales from 2006 to 2015, the product annual compound growth rate of 37.9%. The drug breakthrough global sales of $5 billion in 2014, into the global top ten best-selling drug, no. 10 in 2015. According to the sales revenue in the third quarter of 2016 data, the drug lenalidomide sales growth rapidly, the product is expected to more than 2016 global sales of $2016. According to its annual report predicts that the drug lenalidomide global annual sales will double within five years, to 2020 annual sales or as much as $10 billion.
Domestic: double heron one step ahead
From the domestic market sales sample hospitals in nearly three years, the drug lenalidomide 2013 annual sales of 1.52 million yuan, 2014 annual sales of 10.68 million yuan, 2015 annual sales of 13.38 million yuan, the market base is small, fast growth. At present the original corporate America its drug lenalidomide at the market cultivation stage, the future market needs further depth of mining.
On the domestic market at present the multiple myeloma treatment mainly yeung sum of boron for m (jose carreras) and the United States to the degree of its amine (beauty), two products are now in the patent protection period. On domestic clinical multiple myeloma treatment, boron is mainly used for first-line treatment for Mr M, drug lenalidomide is mainly used in second-line treatment.
Drug lenalidomide is approved by the United States its 2013 into China, commodity called "beauty", formulations for the capsule, specifications have 15 mg and 5 mg, 10 mg, 25 mg four. The CFDA database search, the current domestic filing drug lenalidomide capsule and the company more than ten, main dosage form drug lenalidomide capsules and benzene sulfonic acid drug lenalidomide, tablet declaration enterprise for michele in tianjin science and technology development co., LTD. Drug lenalidomide capsule at many companies, according to the production enterprise mainly have a double heron of Beijing pharmaceutical, jiangsu howson pharmaceutical, pharmaceutical, qilu pharmacy and so on more than a dozen zhengda shine.
Double heron pharmaceutical drug lenalidomide capsule in the short term is still in the leading stage, compared with the subsequent generics have certain advantages, and are on speed up the application directory, and other enterprises products listed road is long, the next drug lenalidomide would not be too fierce market competition. The product such as stock market debut for double heron pharmaceutical product line will be a major breakthrough, relying on lower priced rapidly expanding market, is expected to become the support of the company's sustained high growth potential.
viread
In the Chinese compound patents expire time in July 2017
Global: for two consecutive years sales of over 1 billion dollars
Tenofovir gilead, developed by the United States, is a new type of nucleotide reverse transcriptase inhibitors, 2001 approved by the FDA as a treatment for HIV/AIDS, 2008 has been the European Union and the United States FDA approved for the treatment of hepatitis b, commodity called "Viread. Tenofovir is geely's star varieties, it is the effective medicine for the treatment of HIV/AIDS and hepatitis b. Tenofovir in second liver turn rate and drug resistance problems compared with the main competing goods adefovir ester, biff and entecavir has certain advantages.
In 2015, according to the world's bestselling drug data statistics tenofovir sales of $1.108 billion, a 4.7% increase from the same period. From the point of sales from 2003 to 2015, the product annual compound growth rate of 5.8%. Global sales of $1 billion in 2014, for $2015 in 1.108 billion, has two consecutive years in the sales of $1 billion products.
Domestic: the imitation of enterprise competitive game
Sample according to the domestic hospital statistics, tenofovir 2012 annual sales of 2.42 million yuan, 2014 annual sales of 9.38 million yuan, 2015 annual sales of 25.09 million yuan, a 167% increase from the same period, 2016 years ago in the third quarter sales have up to 80.02 million yuan, is growing rapidly. Rapid growth of the product is mainly thanks to on May 20, 2016, the state department of pharmaceutical price negotiation as a result, tenofovir monthly drug expenses from 1500 yuan to 490 yuan, or 67%, to become the world's your lowest price indications for the treatment of chronic hepatitis b, it objectively promoted the tenofovir volume on the market in 2016, is bound to lead to drug resistance hepatitis b market change.
Tenofovir in June 2008 by gilead company approved to enter China, commodity called "viread, dosage forms of tablet, the specification is 300 mg. In November 2009, geely, hand in hand with GSK by glaxosmithkline tenofovir drug hepatitis b in the Chinese market promotion. In April 2015, the CFDA viread glaxosmithkline (tianjin) with the approval of the public in China.
In 2013, anhui baker tenofovir approved production of active pharmaceutical ingredients. In November 2016, the chengdu bate pharmaceutical for Norfolk WeiYuan approved CFDA production and tablet, tablet size is 300 mg. Is now declare pharmaceutical companies have board shine, anhui baker biological pharmaceutical, federal pharmaceutical, shandong qilu pharmaceutical, zhuhai rossing pharmaceutical nearly 40 companies, etc. At present, the domestic tenofovir rob imitation enterprises is still in the game.
Sharp ai completed
In the Chinese compound patents expire time in April 2017
Global: market gradually decline after 2011
Aza that developed by bristol-myers squibb company, approed for sale in the United States in June, 2003, called "Reyataz" goods, mainly used for the first-line therapy for HIV infection. The product is the world's first once-daily dosing of protease inhibitors, and other antiviral drugs used in AIDS antiviral treatment, potent and sustained suppress HIV, low resistance, easy to use, low side effect on fat metabolism, etc.
According to the world's bestselling drug data statistics, in 2005, the drug sales were $596 million, 2011 sales peaked at $1.569 billion, annual sales of $2015 and $1.139 billion, down 16.4% compared with over the same period. From the point of sales from 2003 to 2015, the product annual compound growth rate of 5.8%. Aza that has exceeded the 2007 global sales of $2007, 2011 peak, market gradually decline since 2011.
Domestic: sample hospital no sales data
Bristol-myers squibb company, that has been approved in China, the goods is called "ai", formulations for the capsule, specification is 0.1 g, 0.15 g and 0.2 g 3 kinds. The product in the sample hospitals were no sales data. Because our country implements the anti-hiv drugs fixed-point production, centralized purchasing, into the national pharmaceutical reserve, unified distribution, allocation, and step by step in the national disease prevention and control network distribution, therefore, aza that market sample hospitals are reflected in the country. |
