Medical network - on January 12, 2016, the agency for the pharmaceutical industry of the whole supply chain big check across the country, and will continue. Can say, no dead Angle is 360 degrees to strictly check the production, business, sales terminals, medical people. It doesn't, and the agency recently released to production, circulation enterprises.
▍ 16 drug firms shall be ordered to make correction
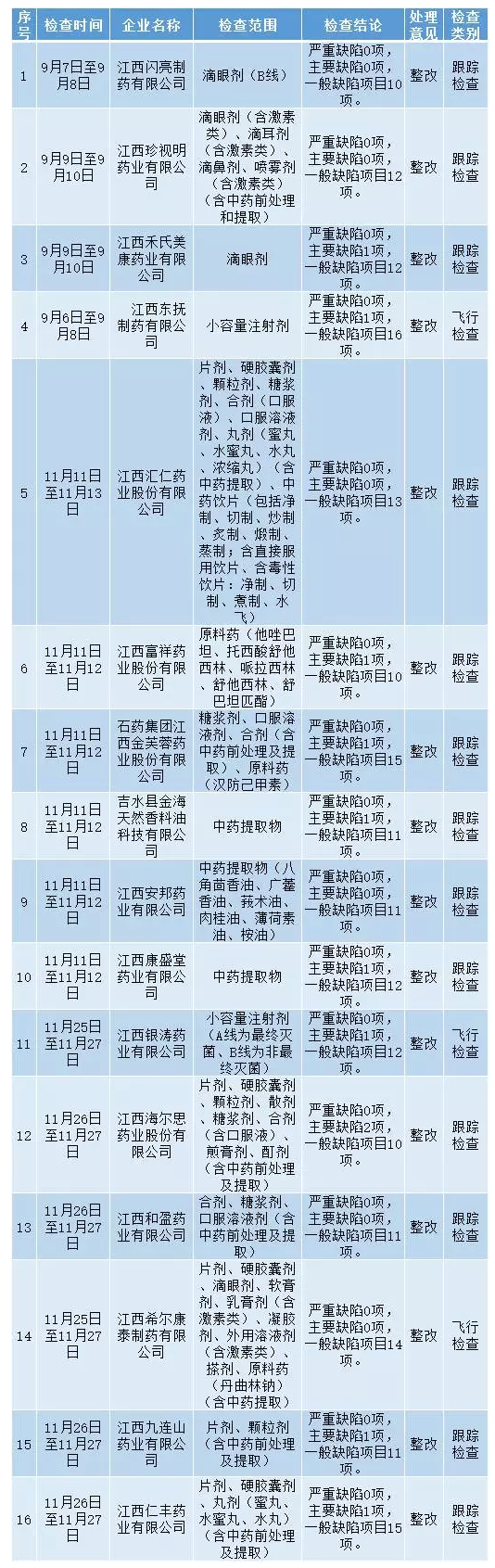
Yesterday (January 11), jiangxi food and drug administration issued the September and December of 2016 drug production supervision and inspection and the processing conditions to no. 4 (2016).
According to jiangxi province food drug administration drug production supervision and inspection plan in 2016, and 2016, 9 to 12 month ShengJu organization team to Jiang Xidong fondle pharmaceutical co., LTD., hill conde Jiang Xiyin tao pharmaceutical co., LTD., jiangxi pharmaceutical co., LTD., has carried on the flight check, to the jiangxi shiny pharmaceutical co., LTD., jiangxi value Ming pharmaceutical co., LTD., etc. 13 pharmaceutical production enterprise has carried on the track inspection, now the inspection found that there are major defects or defects in general.
Reported as follows:
▍ 45 druggist was flying, 43 big problems exposed
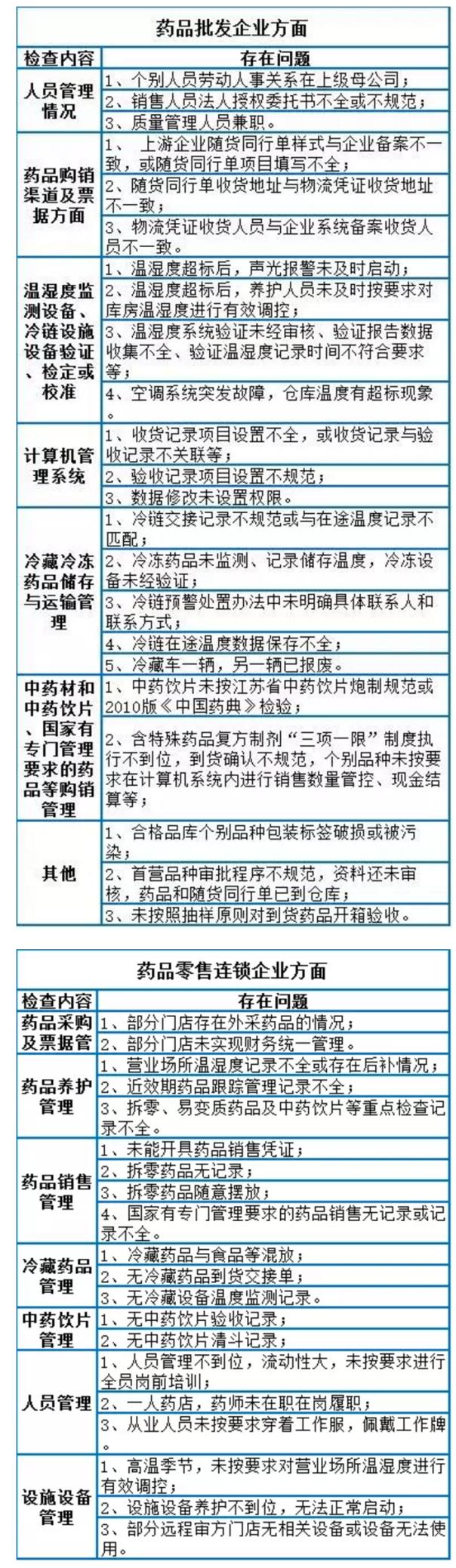
On January 10, jiangsu food drug administration issued "about suzhou pharmaceutical circulation field illegal behavior during the operation of bulletin, the food and drug administration to carry out the previous drug circulation field illegal conduct summarized during the work.
According to reports, the total of 45 check pharmaceutical trading enterprises, including 32 pharmaceutical wholesale enterprises, 13 pharmaceutical retail chain enterprises.
Check adopted different crossover, flight check way, without notice, don't listen to the report, to the work site, direct, good examination results have been achieved. But still found in the centralized checking, part of lax pharmaceutical trading enterprises, a serious violation of drug management quality management standard; A few enterprises ignore the drug laws and regulations and the relevant provisions of alleged illegal activities.
Suzhou food food and drug administration said it would pay special attention to the case clues for verification, supervision and rectification irregularities, strive to improve the regulation efficiency. For alleged illegal case clues organization investigation to verify, in conformity with the case situation, must be filed for investigation by the program, severely punish illegal behavior; Suspected of illegal and criminal, timely transferred to judicial organs investigation and handling. For defects found concentrated examination project, should supervise and urge enterprises to rectification in place within the given time, and to do a good job of supervision and rectification and review of the review. To refuse to improvement or still don't meet the requirements of the regulations, in accordance with the law to make the corresponding processing.
Inspection found that the main problems:
▍ pharmaceutical industry consolidation continues
Around at present, the country power is concentrated, the agency also released successively for pharmaceutical production enterprises, circulation enterprises "fly inspection plan", and very detailed list key check varieties, focusing on enterprise, focusing on link, check way.
In the field of pharmaceutical production, after the state food drug safety administration has released two batches of appointed national drug GMP inspector list again, the number of GMP inspectors have reached 649. With the number of GMP inspectors more and more, in pharmaceutical production enterprise fly under inspection by accept GMP pressure will be bigger and bigger.
In the field of drug circulation, since last year on May 3, national food drug safety administration issued "about consolidation drugs circulation of illegal conduct announcement no. 94 (2016), the provinces have carried out the drug circulation enterprise a clampdown, drug distribution link" card, ticket, zhang, cargo, "one of the important work for the clampdown. For many manufacturers, teamed up with the many ministries and commissions of the state of medicine circulation industry has entered a white-hot, a clampdown scrutiny let enterprise inundated with all sorts of flying.
For across the country and the agency from time to time notified by GMP, GSP drug firms, unqualified drugs list, you don't feel numb. In this round of medicine purge action, who has a chance to be checked, even being knocked down, legal compliance is survival. Look, guess and check large forces are already on the way to check your company! |
