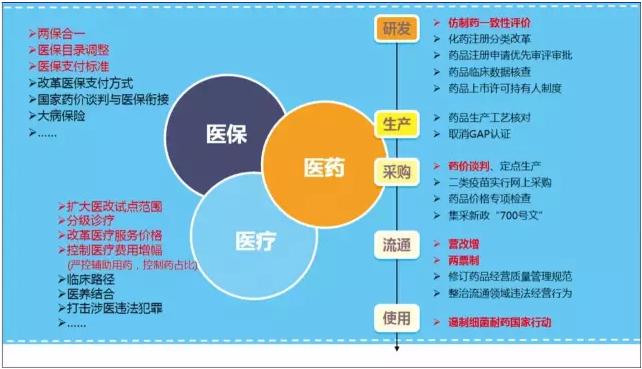
Medical network February 6 - as topics include Chinese medicine policy, in 2016, have done a lot of layout, but in the short term or still hard to see results
Health reform in recent years, certainly cannot leave the "3 d linkage" hot word. Until March 2016, however, that the word is written for the first time the government work report, held many times before, during, and after this as the core of high-level meetings.
High-level push makes "3 d linkage" in the next few years the key to deepen the reform of medical health system, in 2017 the focus of the reform policy and basic all can be classified as "3 d". So, the core of "3 d linkage" related policy currently going? In 2017, which is expected to "standard"?
Health care: five key to progress and to watch
In June 2016, people club department issued by the "about promoting instructional advice on the reform of medical treatment, health care, medical linkage", this is the first time people club department told to promote "three medical linkage", and health care were established in the core position in the "3 d linkage". This is the coverage and medical expenses after payment ratio reached a certain degree of natural result.
In fact, just the past 2016 years, the health care sector released a few larger policy, so the current progress?
"Joining together of two bao"
In January 2016, the state council issued "on the opinions of the integration of urban and rural residents basic medical insurance system", formally put forward to promote urban residents health care and new farming and system integration, urging provinces (autonomous regions and municipalities) before the end of June to the integration of urban and rural residents health care plan and deployment, a concrete implementation plan before the end of December.
From the point of the current progress, the vast majority of provinces (autonomous regions and municipalities) have been issued integration scheme, part of the province (area, city) has completed the integration. Temporarily not issued integration schemes in the provinces (autonomous regions and municipalities), part of the province in fact already integration work, such as jiangsu, anhui, etc.
Catalog, "joining together of two bao" merger, according to the principle of "high not low", the original ginseng protect personnel to use scope, reimbursement levels such as the increase of the whole, and market expansion of opportunities.
On management, most of the provinces with clear right after the "joining together of two insurance" in the social sector except (shaanxi). It also increased the voice in health care, people club department or in the "3 d linkage" some relevant policies to promote more smoothly.
Health insurance directory adjustment
At the end of September, 2016, people club department "2016 national basic medical insurance, industrial injury insurance and maternity insurance drug catalogue work adjustment plan (draft)" for public opinion.
The adjustment of the last directory is in 2009, the adjustment is expected to more than 300 kinds of new drugs.
Currently, adjust the work plan of a formal draft should be already completed, but there is no public net; Directory of selection has been completed, but temporarily haven't released the final version of the directory.
Health insurance payment standard
At the end of November 2016, a club by man and health development planning commission jointly issued by the "about pay of insurance of primary medical treatment drugs standard rules of guidance (draft)". From the news of the 76th session of the national drug trade fair on the BBS, payment standard is expected to be introduced with the new medical insurance directory.
From the perspective of the draft of outflow, payment standard before the complete consistency evaluation, according to the trade name; After the complete consistency evaluation, in principle is formulated according to the common name. Health insurance payment standard reference factor is the main drug actual market value of the transaction. From the pilot areas, the lowest price is the main reference price in the country.
From the point of payment in the draft rules, will cause the hospital drug use is less than or equal to the medicare payment standards.
The medicare payment reform
Medical insurance payment reform has repeatedly appeared in the annual health reform key tasks. Before 2016, around the medicare payment reform mainly do is relatively simple amount in advance. In 2016, the main way of the reform of began to shift to press disease to pay. Current reform of the pilot regions and continuously published clinical pathway can glimpse.
National price negotiations
On May 20, 2016, the first national drug price negotiations, the results of three kinds of negotiations and drug reduction topped out at 67%. Documents require hang around to negotiate drug centralized network and related health policy. After negotiation results so far, has 26 provinces will negotiate drug into all kinds of the compliance costs of medical insurance.
Negotiate price cut into the health care in the future varieties could be more and more.
Medical: deep bedding has become a public medical reform
"3 d linkage" to change the core of the problem is, in fact, health care, namely the reform of public hospitals. From the point of the ultimate goal, is must by "3 d linkage" deepening the reform, and change of public hospitals operating mechanism, change the doctors' prescription behavior.
Health care reform pilot scale
In May 2016, the second batch of comprehensive reform pilot provinces and the fourth batch of health reform pilot cities, including pilot province increased from 4 to 11, 100 new pilot cities, covering the whole country two-thirds of the area. At the end of the news, said the four provinces such as guangdong is expected to be included in the third batch of pilot provinces, then half of the country's provinces are to become pilot reforms.
Classification and treatment
Hierarchical diagnosis is the new focus of 2016, the national health and health conference in August for the first time "grading diagnosis" positioning for first five basic medical and health care system. At the same time, the national health development planning commission issued "notice about advance classification of diagnosis and treatment of pilot work, determine the four municipalities directly under the central government such as Beijing, shijiazhuang city, hebei province, 266 cities as pilot implementation as a pilot city grading diagnosis and treatment work.
In addition, released in June 2016, reform of the state council to handle the signing of promoting family doctor service guidance ", asked 2016 signing in health reform pilot city to carry out the family doctor service; Signed in 2017, the family doctor service coverage rate of 30% or more, key crowd signing service coverage rate of 60% or more.
Establish a family doctor system as one of the key work of hierarchical diagnosis and treatment, can play a key role to the implementation of the final grading diagnosis and treatment.
In the classification diagnosis and treatment in the process of advancing, medical market will be gradually tilted the grass-roots medical institutions, the whole market pattern will be great changes.
Medical fees reform
In the cancellation of drug addition, to reduce the medicine proportion, straighten out the medical service price is one of the most important aspects of the "vacate basket change a bird". In July 2016, the national development and reform commission, planning commission, the agency department, finance department four ministries views to push forward the reform of the medical service price, demand by 2020, gradually establish on the basis of cost and income structure change of price dynamic adjustment mechanism, the basic relationship between the medical service price comparison; Promote the reform of medical service pricing, expanding the scope of the service unit according to disease, according to charge.
In January 2017, the national development and reform commission website on the proceed with the press disease to plant the charge notice, announced in 2011 pilot basis, further expand the scope, diseases is carried out for 320 kinds of fees, and clearly the implementation schedule is given at the end of 2017 - city public hospital comprehensive reform pilot area before the disease is paid by the disease may not be less than 100.
Press disease to plant will cost with the medicare payment reform, will completely drug into medical costs, rather than a source of profit.
In addition, in order to let the medical service price adjustments have more space, the price of drugs may also be compressed further.
Increase of controlling medical expense
For public hospital, control the growth of medical expense has been for many years, the goal of control cost and adjust once a year, in 2016 on the notice as soon as possible to determine the medical expense growth requirements, in the end of 2017 to the medical expense growth below 10%.
Related to this, and control of the medicine proportion (pilot cities by 2017 to 30%), and the auxiliary drug control (file only said "monitored drugs").
To sum up, overall public hospital medical expenses growth will slow further, and in medicine proportion control, cancel the drug addition, straighten out the medical service price policy as a whole, the structure of the medical treatment cost will change. Thus, public hospital medicines market scale will probably negative situation.
Medicine: the policy reform throughout the whole industrial chain
Since 2009 since the launch of the new health care reform have been more relevant reform measures in the field of medicine, has been criticized "reform" change "medicine". But after the policy of drug change more procurement procedures, and recently is across from the research and development, production, procurement, distribution and use of the whole industry chain.
Research and development, "consistency evaluation" refresh
After the inspection of the clinical data of 2015, 2016 for the link of research and development, the theme of the policy is "consistency evaluation" to almost all occupied.
In March 2016, promulgated by the state council concerning the evaluation opinions of generics effect quality and consistency, and later the CFDA successively published 17 key documents.
On December 30, 2016, at the 31st session of the central comprehensively deepen reform leading group reviewed and adopted the "on further reform and improve the use policy for producing and distributing medicines of several opinions, put forward to strictly drug public review of the examination and approval system, accelerate the drug quality and curative effect evaluation consistency, pushing drugs listed pilot license holder system.
In January 2017, the state council issued the central designated place on the third batch of lifted the decision of the administrative licensing items ", including the cancellation of drug clinical trial institution qualification first trial, pharmaceutical excipients (excluding drugs with auxiliary materials and import of medicinal materials) to register for examination and approval. The cancel the drug clinical trial institution qualification first trial is considered to BE the prelude to a clinical trial base for the record management, this will solve the current clinical trial base is difficult to meet the demand of a large number of BE.
About consistency evaluation, the provisions of the state completion time is as follows: national medicine in October 1, 2007 approved chemicals generics oral solid preparation should be completed by the end of 2018 the consistency evaluation; Which need to carry out clinical efficacy test and the variety of special circumstances, should be completed by the end of 2021 the consistency evaluation, outstanding overdue will not be registered again; After the first species through consistency evaluation, other varieties of the same shall be completed within three years.
Released from the current policy and market situation, difficult to finish within the allotted time. As a result, the industry still exists "may not be completed extension" expectations.
But throughout the current deepening the reform of the overall policy, generics complete consistency evaluation is the foundation of other policies, such as medical insurance payment standard, etc., so the determination of promoting the policy is not in question. And, in the backdrop of the reform of the "supply side", generics consistency evaluation is the "capacity" of a sharp object, especially has a precedent in the United States and Japan and other regions. To hold fluky mentality of the enterprise, may be the final outcome will be miserable.
In addition to the consistency evaluation, research and development in 2016 and chemical drug registration classification reform, reform policies such as marketing authorisation holder system.
Procurement - the price pressure is still huge
In June 2016, "about further do a good job of public hospital drug centralized purchasing notice (draft) quietly out of the country, to fix around in the execution process of no. 7, no. 70, it can peep medicine mining policy developments:
Direct net drugs autonomous hanging nets, autonomous quotation;
Promote national price negotiation result and health policy cohesion;
Put an end to place protection;
Proprietary Chinese medicine exclusive varieties pilot provincial joint procurement;
The distribution, promote two votes;
Formed by the end of public resources trading platform of medicines and chemical reagents, consumable purchase integration work plan...
With the expansion of pilot cities, drugs, there are more and more of the municipal bidding procurement, and various forms, such as Shanghai and shenzhen GPO, ningbo mode, sanming alliance, etc.
In short, in the purchase link, the price pressure is still huge.
Circulation - "two votes" met "camp to increase"
"Camp in the field of circulation to increase" and "two votes" is becoming a hot spot in 2016, especially the latter.
In 2016, the national post many times of two votes. In January 2017, the reform of the state council office and the planning commission and other seven ministries jointly issued the "on drug procurement in the public medical institutions in the implementation of the implementation of the" two votes "opinion (try out)", and reform pilot city for comprehensive reform pilot province took the lead, encourage other areas implement the "two votes", for the 2018 fully implemented throughout the country.
In addition, in the former mentioned "on further reform and improve the pharmaceutical production circulation use policy several opinions also has" the drug distribution "two votes" reform, reduce the circulation link "of the related content.
So far, no any information in addition to guangxi, Beijing, revealed to the meaning of two votes, other provinces are more or less have revealed to introduce two votes.
Except for fujian had been executed in provinces to promote two votes as follows: on November 1, 2016, anhui officially adopted two votes; On December 15, 2016, implement of two votes in qinghai province; On January 1, 2017, shaanxi, hunan will formally implement two votes; On May 1, 2017, hebei officially adopted two votes; June 1, 2017, chongqing officially adopted two votes.
In addition, in November 2016, the shandong inform clearly put forward that in dongying, weifang, jinan, Qingdao, weihai, city of binzhou six will steadily promote drug purchase of two votes. In August 2016, hubei province, wuhan city, xiangyang city, ezhou, three cities of the implementation of two votes.
Ningxia and sichuan have draft (discussion paper), but the file for the implementation of the "two votes" time has passed, a formal draft released temporarily not seen, should have been delayed. Zhejiang, guangdong, jilin, jiangsu, etc are put forward for two votes, hainan, liaoning, shanxi, jiangxi, henan, yunnan, Inner Mongolia are mentioned in the public hospital reform pilot city of two votes.
For manufacturing enterprises, the "two votes" meets "camp to increase", compliance costs will be greatly increased; For circulation, increase of concentration of industry has already become inevitable, transformation of large circulation enterprises will become the CSO or disappear (by mergers and acquisitions, etc.).
Conclusion > > >
"3 d linkage" constantly improve, will change the pattern of China's current medical and pharmaceutical market, the patient and the market will be gradually to the basic flow, the flow of the process must follow market differentiation, and may with the narrowing of the size of the market; For production and circulation enterprises, the overall trend is toward the direction of the "small profit" and "high concentration".
In addition, in the process of "3 d linkage", part of the policy will also be prompted prescription from hospital to the retail sector, and this is another big topic of Chinese medicine market. |
