| Micro-channel |
 |
|
|

|
|
|
| |
| What is the amount of new drugs and double dividend? The status of clinical self-examination variety listed |
| |
| Author:中國銘鉉 企劃部 Release Time:2017-8-4 10:23:43 Number Browse:836 |
| |
The medical website on August 4, 2015 began the clinical data of 22 July 2015 self-examination, has been over two years. On July 21, total bureau of national food and drug supervision and administration of the food and drug review inspection center (hereinafter referred to as "CFDI") announced the drug clinical trial data check periodically report (July 2015 - June 2017) ", the drug clinical trial data for two years the inspections conducted a periodic summary.
The variety in the inventory is different. Among them, the import of new drugs and dual-report drugs is widely believed to be good, and the attention of the industry. So in particular, what are the qualities of a truly positive variety?
New drug to be imported: the main bright spot of new drug in 2017
The rapid approval of new drugs for new drugs has been added to the success story. In July 2017, the sodium sands of nohua was approved for import. From March 2016 to declare the import market, in the same year in August to 2016 check list (the third batch of inspection) listing announcement no. 142, in December for priority review, nearly a year and a half time, sand kubah qu valsartan sodium piece is approved by the import market. Although it is not available for import approval in the national import and drug database, this is another successful case for the rapid approval of imported new drugs in 2017.
According to table 1, 15 products - enterprises are listed for the first time, 9 of which are imported products from foreign companies. In March 2017, administration of inviting public administration of the state food and drug supervision and administration of imported drug registration management related matters about adjusting decision (draft) "opinions of notice, on June 19, 2017," imported drug registration certificate issued by the examination and approval items (traditional Chinese medicine, natural medicine, biological products) service guide ", a new global drugs import approval will be 2017 CFDA new listed several of the main window.
Of the six approved products in China, 3 are biological products, 3 are chemical generics, and 3 chemical generics are oral preparations of fumarate tenofovir dipyridrol. Domestic products entering the list of domestic products are still based on biological products, and the approval of drug generics is not optimistic.
In 2017 has been successfully transfer approval data can be found in the trusted Jiang Xiji people from liaoning huarui joint Pharmaceutical mig column nai 5 mg calcium tablet and 10 mg two approval, mig column nai calcium tablet currently listed manufacturer number within five, only Beijing sihuan division in clinical inspection check Pharmaceutical Co., Ltd. To survive in 2015 approved by the public, hainan, zhongtian Pharmaceutical Co., Ltd., zhejiang huahai Pharmaceutical Co., Ltd., guizhou cheonan Pharmaceutical Co., Ltd., and a working Hicin Pharmaceutical Co., ltd. is withdraw the application, no generic manufacturers listed within two years. This case reflects the delay in the approval of generic drugs. The approved products can continue to receive preferential policy thresholds. In the short term, the products of manufacturers can still be good business.
In addition, the import of expiration of the original drug distribution rights to sell to large commercial circulation background of domestic enterprises or foreign companies of similar product line phenomenon also staged: Pfizer will accept it and bristol-myers squibb, joint development of pp shaaban piece of rights in China, China resources san-jiu responsible sanofi global over-the-counter one of the flagship product is good after distribution and promotion in the Chinese market.
Table 1 is the first batch of listed products - enterprise list in 2017
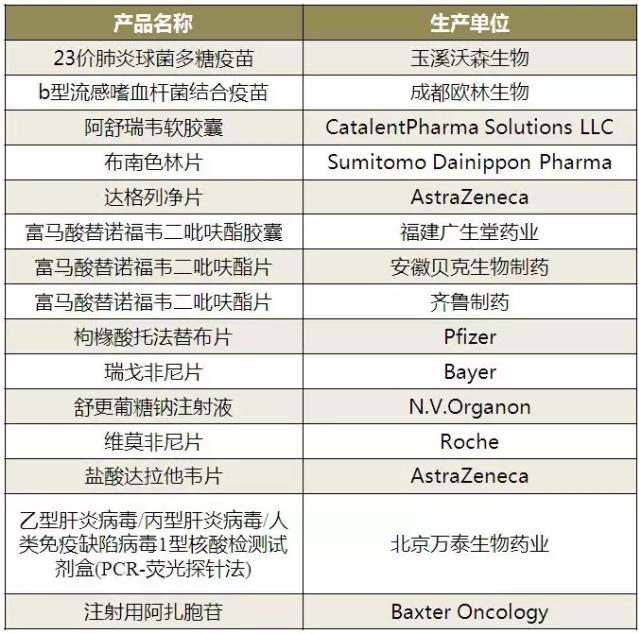
(data source: Brenda data V3.2)
"Double report" : the registered activity of the injection is high
In terms of conformance evaluation, the "double -" companies - products that have been listed in the United States have been approved at a rate not as fast as the approval rate for new drugs.
Domestic pharmaceutical production enterprise has been approed for sale in the European Union, the United States or Japan generic drugs, according to the work plan for the reform of release chemicals registration classification of announcement (food and drug supervision bureau announcement no. 51, 2016) for generic drugs registration related requirements of the declaration, review by the human medicinal center, as through consistency evaluation after approved. Through the variety of conformance evaluation, CFDA announces to the society. The enterprise may label the internal evaluation and the evaluation in vitro in the drug specification and label.
As shown in table 2, hainan split pharmaceutical co., LTD., injectable azithromycin 2012 annual report is produced, in October 2015, the FDA approved production temporarily, in November 2016 in domestic priority review, has not yet approved, also failed to pass the consistency evaluation of identity.
"Double quote" not all the products has launched the registered declaration, such as huahai paroxetine tablets, bupropion hydrochloride zyban, that split ketoneses, doxycycline hydrochloride zyban, ogilvy sand jotham ester hydrochlorothiazide tablet and hydrochloric acid degree rossi with enteric capsules, Shanghai AnBiSheng bupropion zyban, etc., did not start the registration in China.
Table 2 "double newspaper" enterprise - product priority review status
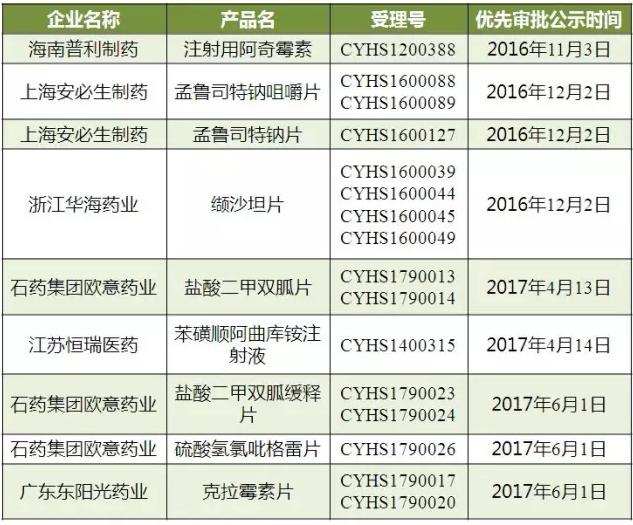
(data source: Brenda data V3.2)
On June 9, 2017 administration office open for the quality of generic drugs and curative effect evaluation to accept the consistency examination guide (consistency evaluation varieties) (draft) "and" the quality of generic drugs and curative effect evaluation to accept the review guidelines consistency (within the territory of collinear production and market in Europe and the United States, variety) (draft) "and the relevant documents, means that the" double quote "line of products will be approved by is expected in the second half of the consistency evaluation of identity.
According to the analysis of the present domestic and ANDA "double newspaper" products, the company has found that the products of the listed injection in China are more than the same. The re-evaluation of the injection will be started and it is estimated that the listed injection will be completed in about 5 ~ 10 years. Conducted by the state council general office on 2016 generic drugs quality consistency and efficacy evaluation opinions ", "chemical classification of new registered approval prior to implementation of generics, who is not in accordance with the principle of in line with the original drug quality and curative effect of examination and approval, shall be carried out consistency evaluation" including the injection.
This means that the same variety of other drug production enterprises should complete the consistency evaluation within 3 years once the "double - report" injection is approved. If overdue, no re-registration, the other drug manufacturers concerned will speed up the consistency evaluation.
For manufacturers at home and abroad are listed, "double quote" regarded as through consistency evaluation of drug varieties, and social security ministry goalkeeper in medicare payments to appropriate support, medical institutions should be prior purchasing and preferential treatment in clinical, dividend policy.
With the production enterprises of the same variety of drugs reaching more than 3, the health and family planning departments will no longer select the varieties that have not passed the conformance evaluation. According to table 3, many of the products that have obtained ANDA are two. Domestic enterprises need to pay attention to the OTC reporting channels of FDA in the United States. The channel is relatively convenient, such as ibuprofen tablets, which are now adopted by the manufacturers of zhongshan anshi and dalian merlot.
"Double quote" oral medications most is its state in the declaration, the current domestic listed registration filing and consistency evaluation to different department is in charge, unlisted products can priority review and finally won the award for the evaluation of consistency, it remains to be the CFDA streamline processes.
Table 3 sales volume of "double - report" products listed/declared status quo
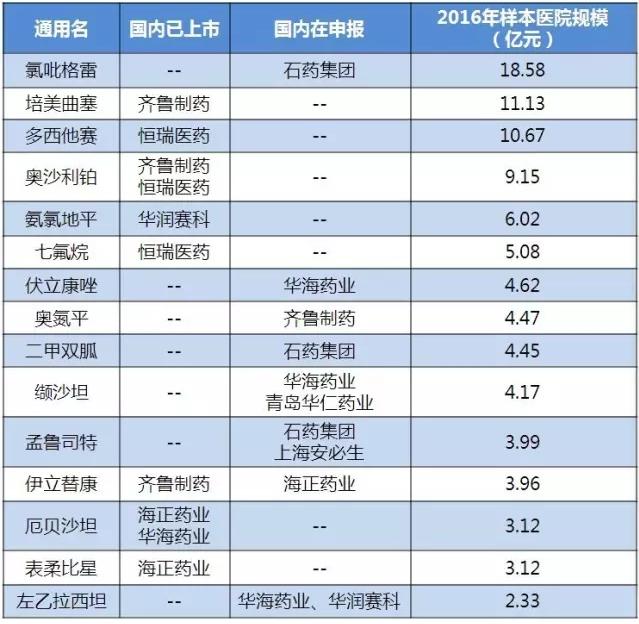
(data source: Brenda data V3.2)
Summary > > >
Clinical comprehensive verification, 2033 to accept order by CFDI the drug clinical trial data check periodically report (July 2015 - June 2017), written only 146 to accept order, among which written announcement no. 117 in 2015 to accept only 2 accept order. This means that 90% of the approved list will be approved, and the remaining small amount will be approved by the end of 2017.
Judging from the current approved, generic drugs for oral administration approved quantity is less, declare "success rate is low, in the short term is expected to be approved but only to a double quote products, but when can the listed remains to be seen CFDA progress next. The domestic manufacturers of the listed chemical products will continue to enjoy the policy dividend.
|
| |
Previous article:Shortage of drug control! Hospital, pharmaceutical data is due
Next article:What is the amount of new drugs and double dividend? The status of clinical self-examination variety listed
|
| |
|
|
