| Micro-channel |
 |
|
|

|
|
|
| |
| In the field of drug circulation, the second half of the "serious play" is staged, and the focus of enterprises should be seen from the high frequency defect item |
| |
| Author:中國銘鉉 企劃部 Release Time:2017-8-11 8:47:23 Number Browse:663 |
| |
Medical network - August 10, 2017 in the first half of the total cancellation of guangdong province pharmaceutical wholesale (chain) enterprise GSP certificate of 35, 220 rectification within a time limit, the cancellation of pharmaceutical wholesale (chain) enterprise 40 pharmaceutical trading license. It can be seen that guangdong food and drug administration has effectively cracked down on illegal drug circulation.
The author thinks that during the first half of this year, the flying test was only "rehearsed" and the "heavy play" was in the second half of the year.
First half summary
In order to facilitate everyone meet the inspection, the author of the first half of 2017, guangdong food and drug administration announced (revocation of 19, rectification within a time limit 37) check the defect summary analysis, found that was ordered to make correction of the main problems of enterprise specific such as table 1.
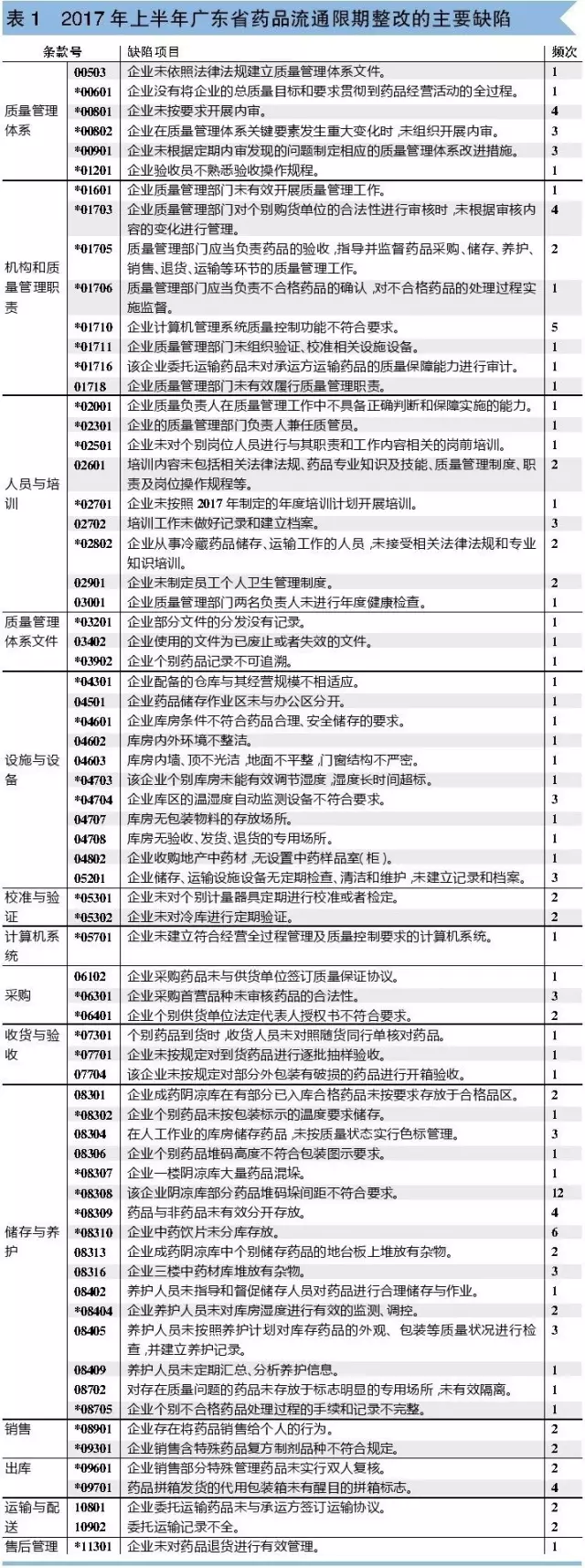
The author found that the three defects of the highest frequency were: 1. * 08308. 2. * 08310 Chinese medicinal materials and Chinese medicine yinpian undivided storage; 3. * 01710 enterprise computer management system quality control function does not meet the requirements.
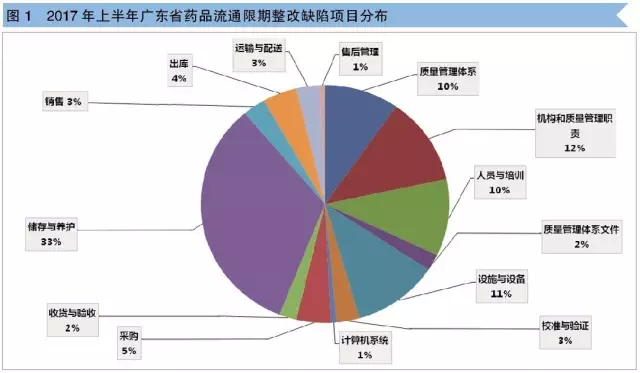
Figure 1 shows that the maximum number of defects in storage and maintenance is 33%; The following are institutional and quality management responsibilities (12 per cent), facilities and equipment (11 per cent), staff and training (10 per cent) and quality management system (10 per cent); In the end, it was procurement (5%), outlet (4%), sales (3%), transportation and distribution (3%), receiving and acceptance (2%), and computer system (1%).
Companies cope with
So how should companies respond to flying inspections? The author thinks that the enterprise should do the following.
The first is warehouse management. 1. Enterprises should invest more and improve their hardware. The warehouse should adapt to the scope of drug operation and scale of operation. If the Chinese medicinal materials and traditional Chinese medicine are used, the warehouse should be equipped with a special warehouse and maintenance work place. Increase the temperature and humidity control equipment (air conditioner, dehumidifier) and temperature and humidity automatic monitoring system. 2. Daily work. The maintenance personnel should pay attention to the monitoring and control of the temperature and humidity of the warehouse. If the temperature and humidity exceed the standard, the processing records should be processed in a timely manner. At the same time, attention should be paid to the use of equipment and daily backup temperature control data. The measuring instruments shall be regularly calibrated or calibrated. The custodian should pay special attention to the stacking of the medicine, including "five distance" "mixing" and "mixing". If the reservoir is short of tension, the mobile sign can be placed. Special administered drugs should be double checked. Attention should be paid to the marked box marks when the box is delivered. The quality manager shall conduct regular inspection of the warehouse, guide and supervise the storage and maintenance of the medicine. At the same time, pay attention to the honest training, strengthen the warehouse personnel's GSP consciousness.
Second, quality systems and documents. 1. The quality management system should be in line with the actual operation of the company. The enterprise shall, in accordance with the new laws and regulations, require inspection and omission, timely revise the system documents, and pay attention to the drafting, revision, review, approval and distribution of relevant records. 2. After the major revision of the quality management system, the internal audit shall be made timely and the internal audit shall be analyzed. 3. Strengthen training. Conduct training according to the annual training plan, record and establish files. Special attention should be paid to the newly revised quality management system documentation training and personnel training for refrigerated drug storage and transportation. 4. Responsibilities of quality management department. Enterprises should attach importance to the status of quality management department. The quality management department should communicate with each department closely and effectively carry out quality management work. Including the approval of the first camp legal qualification to ensure the legality of the purchase and sale channels, timely organize the verification, guide and supervise the quality management of the various links of the drug operation.
Finally, computer systems. In the first half of the test, the outstanding problem is that the enterprise computer management system quality control function does not meet the requirements. Such as drug purchase link lack of control functions, not operating rights to a computer system audit, failed to be responsible for quality management in the computer management system database set up and update. The author suggests that the enterprise should first check the computer quality control function and contact the computer software supplier to deal with the defect timely.
In the second half of the year, the "war" has sounded the horn, and the enterprise only attaches importance to quality management, so that it can be found invincible in the field of flying inspection. (author: CIO compliance assurance organization)
|
| |
Previous article:Chinese medicine injection is afraid of losing its hot selling aura! Antithrombotic pattern rearrangement
Next article:Earthquake emergency, these enterprises have!
|
| |
|
|
