Medical network - on August 15, China's pharmaceutical industry to go abroad, product quality is a hard standard, and the world health organization (WHO) supplier certification (WHOPre - qualification, hereinafter referred to as the "preliminary certification") but also drug firms the quality of a door to the international market.
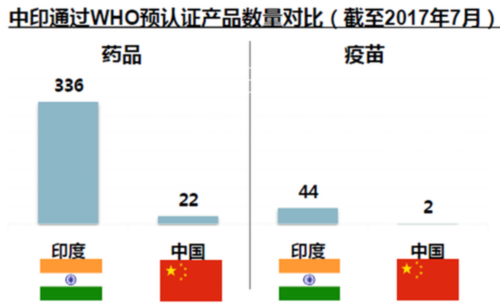
However, so far, has been by the WHO for the certification, can enter the global procurement directory few digital products in China, the number of Chinese drugs and vaccines by the WHO pre certification is only 22 and 2, respectively, than India.
"After the WHO pre-certification, in the case of international markets, companies will be directly exposed to public market procurement, which is a support for developing countries and a promotion for drug companies." Which the world health organization representative office of the expanded immunization JPG Tang Yi project officials told reporters, the first finance and economics "for the domestic market, the first is to have more high quality products, these products through certification, will raise their level of production quality and management level, this also is helpful for the domestic market and its people."
However, national health development planning commission Gao Weizhong, director of the center for international exchanges and cooperation on August 14, said, "in the area along the initiative, China will offer important public products to the world, but we, when it comes to health care and vaccine by the WHO did not have enough quantity in the process of the certification."
WHO has a high threshold for pre-certification?
"By the WHO certification, means WHO's safety and efficacy of the drug/vaccine said, the United Nations procurement agencies can will this drug/vaccine on its procurement list." "Tang yi said.
There is still a gap between China and India in the number of products that have been certified by WHO. India has 336 drugs, compared with 22 in China and 44 in India through WHO, and only two in China.
"The main reason why the gap is so large is that China started relatively late compared to India." Tang Yi said, "from the Angle of enterprises, through the WHO certification is affected by many factors, especially the vaccine the level of the enterprise, we think the main areas of Chinese enterprises is weak in the quality management system, as well as the ability to communicate. In these respects, India is generally higher than China.
The gates foundation Wu Wenda told the first finance and economics, deputy director of the Beijing office of India's drug market is not so big in China, so they start input stage is considered in the international market, doing research and development, the early stage of the product is according to the WHO standards, rather than according to the national standard.
China is the world's second largest drug market, and is a major generic-drug producer, but the only thing China can supply in the world today is its API. It is the Chinese market that is so large that Chinese companies can make a lot of money just by doing good at home markets.
For products that have already received WHO pre-certification, it is much easier to get into other countries, although they are also registered. In fact, after the WHO pre-certification, it is not only to provide quality products abroad, but also to the domestic products.
In tang's opinion, there is another reason for China's low number of pre-certified products through WHO, which is profit. As a global health procurement with a public interest, taking the most money with the least amount of money is a matter of course, but it is not a lack of profit.
"To pass the WHO pre-certified product, the enterprise needs to improve the quality first, which is to invest the cost. It is the cost or the profit, which is also the factor that the enterprise should consider." But on the other hand, large-scale purchases by international organizations, such as international organizations, will bring profits to these companies once the products have entered the international market, according to tang.
Chinese drug companies face cost considerations
In fact, whether Chinese companies to do the WHO certification, it is imperative to have the quality of drugs and vaccines improve it, especially in full swing generics consistency evaluation in China, as well as the national food administration of drug safety to join, for example (international people with drug registration technology to coordinate).
"In order to pass the WHO pre-certification, it is necessary to improve the quality, and in this process, the increase in costs is certain," tang told caijing.
For enterprises, input and output problems need to be considered from beginning to end. Generics consistency evaluation in China in the process of, originally has a lot of product and batch number of the enterprise, has already started to thin body, after all finish a consistency of drug evaluation, 5 million ~ 10 million yuan, the cost of investment is needed.
Food of zhejiang province food and drug administration recently issued the "zhejiang province intends to develop generic drugs not consistency quality and curative effect evaluation of varieties of information", the province has 32 drug firms gave up 160 drugs, no longer a generic consistency evaluation.
"For many companies, generic consistency evaluation need large investment of manpower, material resources and financial resources, have a lot of companies do a batch number to if their products do not have advantage, then in order to improve the quality and put so big is not appropriate." "Says one drug expert.
Improving the quality of drugs or vaccines doesn't seem to be that easy. Wu wenda gives an example: "China's je vaccine received the WHO pre-certification in 2013, and it took nine years before and after, and about 40 million dollars was spent on technical support. More than 400 million doses have been sent to areas outside China. Although the b brain vaccine is late for the WHO pre-certification, it has a significant impact on public health.
The process of taking the b brain vaccine for WHO's pre-certification has come a long way, suggesting that China's vaccine industry is at a distance from international quality standards. However, the case also shows that the Chinese medicine industry is not really far away from the world.
"Compared with India, in terms of hardware, we have no poor, poor is known as the software, including the file system, for a good understanding of the specification and implementation, quality, etc., this is common problem of Chinese enterprises." "Tang yi said.
|
