| Micro-channel |
 |
|
|

|
|
|
| |
| Imported medicine, hemophilia domestic varieties are under threat |
| |
| Author:中國銘鉉 企劃部 Release Time:2017-8-18 11:17:05 Number Browse:878 |
| |
The second batch of drug negotiations in the medical network on August 18, the drug for the treatment of chronic diseases in the treatment of high - priced rare diseases. Gene recombination technology giant novo nordisk restructuring of clotting factor Ⅶ will be only a hemophilia treatment, negotiate a price cut into the national health insurance directory, patient populations to obtain real benefits, industry competition to boom.
Novo nordisk restructuring of clotting factor Ⅶ a for clotting factor Ⅷ or Ⅸ inhibitor > 5 bu congenital hemophiliac, at the same time is acquired hemophiliac, congenital human recombinant coagulation factor Ⅶ a lack of medicines, also to have the platelet glycoprotein GP Ⅱ b - Ⅲ a and/or HLA antibodies and poor form now on platelet transfusion is invalid or platelet weakness, commodity called "its".
Through negotiation, the specification of 1 mg (50 kiu)/Ⅶ a clotting factors and the restructuring of the people by the original bid the lowest price 6350 yuan to 5780 yuan, a 8.98% decline, this is the second batch of negotiations by minimum import drugs, into the medical insurance directory is the key step, not only make the hemophilia groups solve after all at his own expense, the difficulty of your, medication, medication, and it will lead to hemophilia treatment market reshuffle.
The domestic hemophilia market is over 2 billion
Hemophilia is a hemorrhagic genetic disease that affects the quality of human life. There is no cure in the world. Although hemophilia is a rare disease, no medicine can cure disease, but not by anthropogenic sex of exogenous additions or restructuring clotting factors, can reduce bleeding or bleeding hemostatic, prevent poor prognosis.
Is fully management and are almost 75% of the patients with hemophilia treatment, blood Chinese friend's home, according to data from 100000 ~ 130000 Chinese patients with hemophilia, constitute a relatively large scale market, has been highly appreciated by the world health organization.
Hemophilia is divided into a, b, c three types, respectively, the lack of clotting factor Ⅷ, Ⅸ and Ⅺ. The incidence of haemophilia is one in 10,000, the incidence of hemophilia is one in 35 thousand, and patients with type c are rare. Hemophiliacs are almost male, and women generally do not develop the disease, but they can carry the disease-causing genes that lead to a 50% chance of developing males. Party a and b both hemophilia performance may as atavism, clotting factor is the most common Ⅷ defects lead to the lack of or function with hemophilia a.
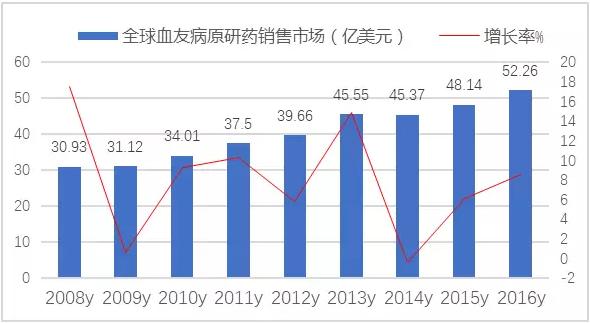
In 2016, the market for global hemophilia was $52.26 billion, up 8.56% from the previous year. Main branded product restructuring Ⅷ clotting factors, blood coagulation factor Ⅶ a, recombinant coagulation factor Ⅸ, restructuring Fc fusion protein and blood coagulation factor Ⅸ.
Figure 1 the market for the market of global hemophilia
On June 15, 2014, the Chinese medical association hematology branch issued the 2014 China expert consensus on the diagnosis and treatment of sexual hemophilia. Hemophilia drug therapy mainly is coagulation factor and hemophilia support hemostatic medication. Clinically used to treat hemophilia people Ⅷ clotting factors, human recombinant coagulation factor Ⅷ, restructuring Ⅸ clotting factors, restructuring Ⅶ clotting factor a and prothrombin complex, as well as hemophilia support medication desmopressin, tranexamic acid, amino caproic acid, thrombin and antibody immune drugs have entered the 2017 "national basic medical insurance, industrial injury insurance and maternity insurance drug catalogue.
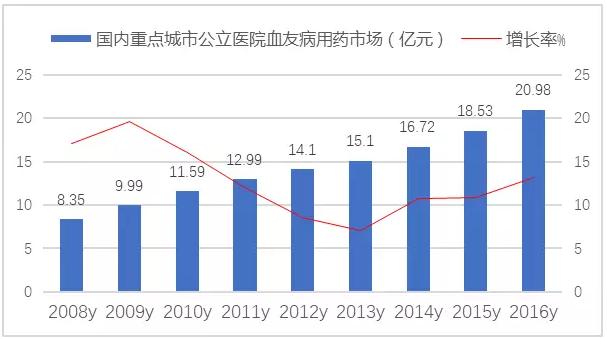
In 2016, hemophiliosis of hemophilia in public hospitals in key cities of China was more than 2 billion yuan market scale, which was up 13.32 percent year on year. The current situation of the supply and demand of coagulation due to subclass drugs, and the rapid growth of coagulation due to subclasses have been paid attention to by the government departments.
FIG. 2 the market of hemophilia medicine in public hospitals in key cities in China
Human recombinant coagulation factor Ⅷ medicare to relax
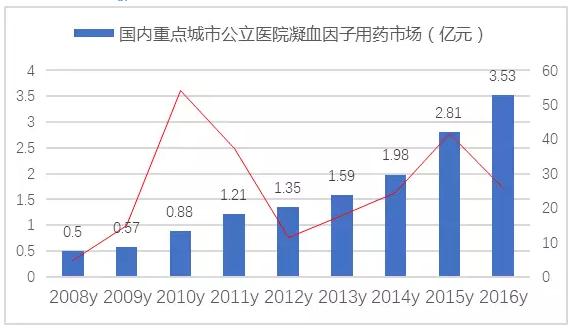
Domestic licensed clotting drugs have human recombinant coagulation factor Ⅷ co-stimulating, restructuring Ⅸ clotting factors, restructuring clotting factor Ⅶ a, man Ⅷ clotting factors and the five prothrombin complex products, 12 companies compete for the market at home and abroad. In 2016, the market of coagulation of coagulation in domestic urban sample hospital exceeded 350 million yuan, up 25.71% year on year.
Figure 3 the market of coagulation factors in public hospitals in key cities in China
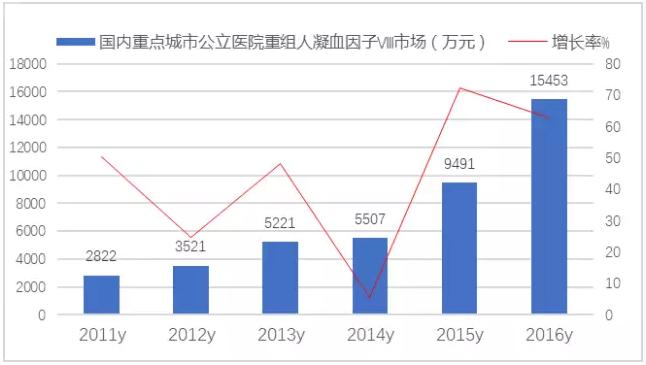
CFDA approved import human recombinant coagulation factor Ⅷ is best for check of the Austrian Baxter AG (Advate), the United States Bayer HealthCare LLC, worship the sarkozy (Kogenate R FS), the United States Wyeth Pharmaceutical Inc Ren Jie (Xyntha). 2016 domestic city hospital restructuring clotting factors were Ⅷ drug amount is 154.53 million yuan, a year-on-year growth of 62.82% a year.
Figure 4 key cities for the domestic public hospital restructuring clotting factor Ⅷ drug market
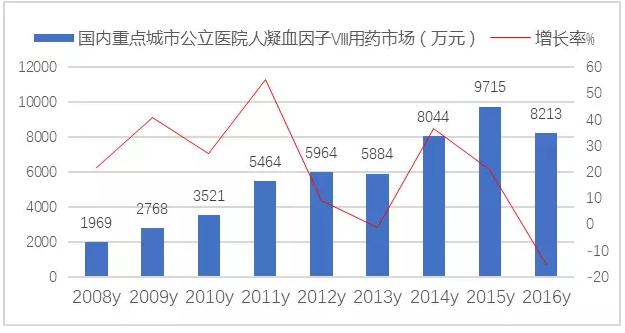
Human recombinant coagulation factor Ⅷ belongs to gene recombination technology development of plasma source of clotting factors, is leading varieties of hemophilia at home and abroad market. Clinical used in swine hemophilia patients with congenital absence of clotting factor Ⅷ control and prevention of bleeding. 2017 medicare catalog for restructuring of clotting factor Ⅷ use policy loosening comprehensively, the original limit no blood Ⅷ factor serious bleeding in hemophilia people use, instead of children with hemophilia a, as well as the use of adult swine hemophilia hemorrhage, increase the clinical use and market-oriented development process.
Human recombinant coagulation factor Ⅶ scale of hundreds of millions of a market
Human recombinant coagulation factor Ⅶ a is developed and produced by Novo nordisk biological engineering drugs, called Novo Seven goods, it is mainly used for preventive treatment of hemophilia group because of clotting factor Ⅷ or Ⅸ inhibitor > 5 bu lead to bleeding, and is expected to inject blood coagulation factor Ⅷ or blood coagulation factor Ⅸ congenital hemophiliac, with high memory response and congenital F Ⅶ deficiency patients safely and effectively stop the bleeding. Human recombinant Ⅶ a clotting factors is the guarantee of hemophilia community survival, is the world's main varieties of clotting co-stimulating drugs.
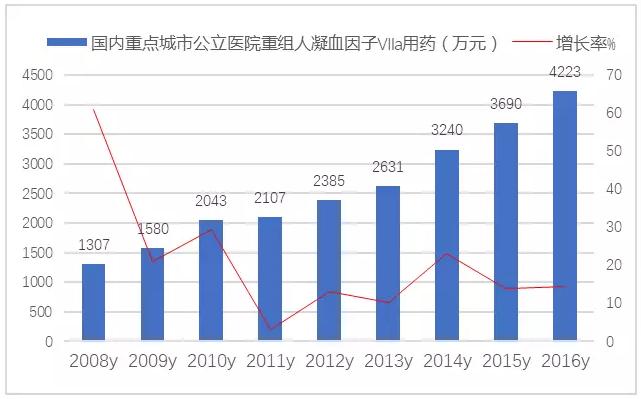
In 2002, human recombinant coagulation factor Ⅶ into market in our country, a commodity called it, is the domestic exclusive varieties. In 2016, the market of domestic urban sample hospitals was 4223 million yuan, up 14.44 percent year on year. Domestic terminal use nearly 100 million yuan market scale, 2017 people club department to negotiate with novo nordisk after cut into the health care, solved the difficult problem of hemophilia out-of-pocket medical group, will also promote human recombinant coagulation factor Ⅶ a market growth.
Figure 5 key cities for the domestic public hospital restructuring people clotting factor Ⅶ a drug market
Domestic clotting factor Ⅷ market declines
Is domestic production of clotting factor Ⅷ blood extract freeze-dried biological products, while clotting factor Ⅷ also life-saving medicine for the treatment of hemophilia, however, in the domestic plasma under the situation of serious shortage, has the tight supply in the coagulation factor Ⅷ, and to avoid potential sources of blood virus problems, for security reasons, people Ⅷ clotting factors is not the only option.
At present, the production approval from the CFDA clotting factor Ⅷ manufacturers, a total of eight, alex blood products in Shanghai, chengdu rong raw pharmaceutical co., Shanghai emerging pharmaceutical co, bio-pharmaceutical, shandong tai bang biological products the same way, the green cross biological products (China), national medicine group Shanghai blood products, made by hualan bio-engineering engineering co.
In 2016, the domestic city hospital blood coagulation factor Ⅷ market is 82.13 million yuan, compared with last year fell 15.46%. Competition shows that anhui green cross (China) biological occupy 43.75%, made by hualan bio-engineering occupy 19.07%, Shanghai alex occupy 19.05%, shandong tai bang biological occupy 18.13%, of which made by hualan bio-engineering and Shanghai alex compared with last year fell 4.98% and 4.98%.
Figure 6 key cities for the domestic public hospital blood coagulation factor Ⅷ drug market
Human recombinant coagulation factor Ⅷ, restructuring Ⅸ clotting factors and restructuring clotting factor Ⅶ a three drugs into the 2017 version of the health insurance directory, show that the recombinant coagulation factor drug levels have greatly increased, on the one hand, ease tensions in the clotting factor market, objectively makes blood clotting factor Ⅷ face challenges.
The prothrombin complex foreground cannot be underestimated
Prothrombin complex (PCC) commonly known as Ⅸ clotting factors and its organization composition containing Ⅱ clotting factors, Ⅶ, Ⅸ, Ⅹ. Is mainly used in the treatment of hepatitis b prevention hemophilia and because of the blood coagulation factor Ⅱ, Ⅶ, Ⅸ, Ⅹ out of disease caused by a lack of hemostasis and clotting factor to have Ⅷ neutralizing antibodies have preventive treatment hemorrhage patients with hemophilia a.
B hemophilia is caused by blood coagulation factor Ⅸ lack of blood coagulation function disorder, accounts for about 15% of the number of hemophilia, less than a (hemophilia. Prothrombin complex could supplement clotting factors, through the bypass activated form F Ⅸ a: Ⅶ a compound, can make patients in local lesions form live clotting enzyme, promote blood clotting, effective hemostatic effect.
In 2016, the market for the thrombin complex was 42,530,000 yuan, up 22.56% from the previous year. In terms of market share, hualan organism occupies 57.87%, shandong taibang biology occupies 22.59%, Shanghai emerging medicine occupies 14.64%, guizhou taibang biology occupies 4.87%.
2012 CFDA approved wyeth vial recombinant americans clotting factor Ⅸ registered in China, commodity called bei fu (BeneFIX), bring varieties of domestic competition, medical insurance directory has closed in the 2017 version, people prothrombin complex market prospect to be reckoned with.
Summary < < <
With the improvement of the medical security system and to raise the level of clinical diagnosis and treatment for patients with hemophilia more care and support, to speed up the present research and development production of clotting factor for help and support use has very positive meaning. |
| |
Previous article:Half the pills! How will the clinical path of drug companies respond?
Next article:The proportion of single drug stores fell to 50.63 percent, and the retail terminal 3 major new trend
|
| |
|
|
