The medical network, on August 24th, has so far been a medically intractable disease.Cerebral apoplexy is a disease that causes brain tissue damage due to sudden bursts of blood vessels or blockages in the brain that cause blood to flow to the brain.Clinical antiplatelet coagulation, anticoagulation, thrombolytic, neuroprotective and hypotensive therapy are the main means of treatment.
After the introduction of the national health care catalogue in 2017, many anti-thrombotic drugs entered the insurance system and the policy of the people was further implemented.After new drugs and cutting shaaban, darby and ester group, on behalf of Greg lowe, pp shaaban, ShuLuo,, music class, for the peptide and indole Finn and up to eight new products into the liver decyl health b, under the drive of rigid demand, will reshape the antithrombotic market pattern in China.
Global anti-thrombotic drugs are hotly contested
In recent years, the global anti-thrombotic drug has received a bumper harvest.In 2015, the U.S. FDA approved two new antithrombotic drugs, the first triad Edoxaban, Savaysa, and the American Madison pharmaceutical company, Cangrelor, Kengreal.At the same time, the FDA also approved the fourth indication of boehringer's darbiaga group of dimethylsulfonate, which is the prevention of deep venous thrombosis and pulmonary embolism.Thus the global anti-thrombotic market has added new active factors.
In June 2017, the FDA approved the Xa factor inhibitor Betrixaban capsule (Betrixaban, Bevyxxa) of Portola biological co.This is the fourth direct Xa factor inhibitor of anticoagulant therapy after levvaroxan, alpazaban and indosa.The prevention of complications of venous thromboembolism (VTE) for adult acute internal medicine is also the only therapeutic drug to replace warfarin, which provides new options for the treatment of atrial fibrillation anticoagulation.
In 2016, according to multinational pharmaceutical companies earnings from global antithrombotic branded drugs may need sales of $17.559 billion, 13.57% year-on-year growth, antithrombotic markets around the world have reached $23 billion in the size of the market, fuelled by listed on the new drug of the world's high-profile 10 drug competition (see table), predict 2018 will hit a record high of $30 billion.
Table 1:10 antithrombotic drugs approved by the FDA in recent years
Antithrombotic drugs enter health insurance and domestic market is shuffled
In the rapid development of the global treatment market, CFDA has successively approved the Pradaxa capsules (Pradaxa).Tai bi quan), bayer's rivastaban (Xarelto;Brilinta for astrazeneca.) (Eliquis), bristol-myers squibb/Pfizer.Alecital is registered in the country, and these drugs are rapidly gaining market after listing, which objectively promotes the growth of the anti-thrombotic market in China.
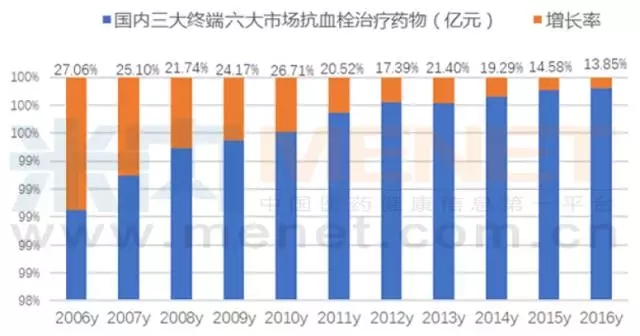
According to m Intranet data show that in 2016 the domestic urban public hospitals, public hospitals at the county level, the urban community center, towns and townships, physical pharmacy, online pharmacies three terminal six big market antithrombotic western consumer terminal reached 18 billion yuan, year-on-year growth of 13.85% a year.According to industry analysis data, under the promotion of Chinese and western medicine, the total drug use of anti-thrombotic drugs in China has reached the market size of 275 billion yuan.
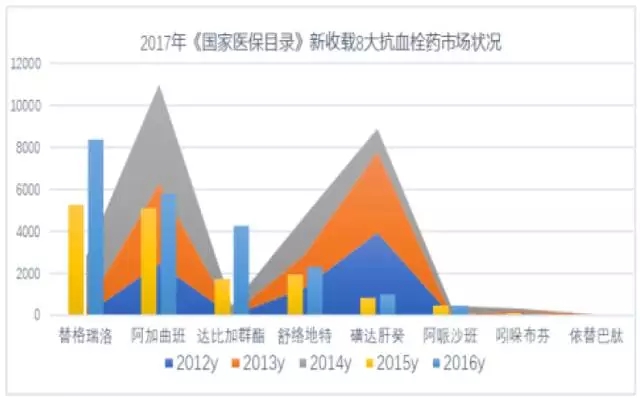
After the second batch of negotiations in 2017, tigreelow went into medicare.In 2017, there are eight new drugs for the anti-thrombosis of health insurance, and 31 varieties of western medicine are available.Formed a platelet aggregation inhibitor, direct factor Xa inhibitors and direct thrombin inhibitor, heparin, enzymes, vitamin K antagonists and other resistance to antithrombotic drugs such as seven small classes, will lead to a major change in the antithrombotic market pattern, domestic antithrombotic drugs in 2017 is expected to exceed 2017.

Antithrombotic TOP5 drugs account for 80 percent of the market
According to m Intranet data show that in 2016 key cities for the domestic public hospital antithrombotic drugs TOP5 clopidogrel, low molecular heparin and cutting shaaban, aspirin, bei other drug amount reached 2.75 billion yuan, account for 80% of the public hospital antithrombotic drug market.
In the new anti-thrombotic drugs, drugs such as rivaroxaban, bepropion, tigerrillo, dabiaglio, bivaruda, and apperacaban increased by 46 per cent year on year.Rivastabant is the leader of a new generation of antithrombotics.
A new generation of antithrombotic drugs led by livastaban
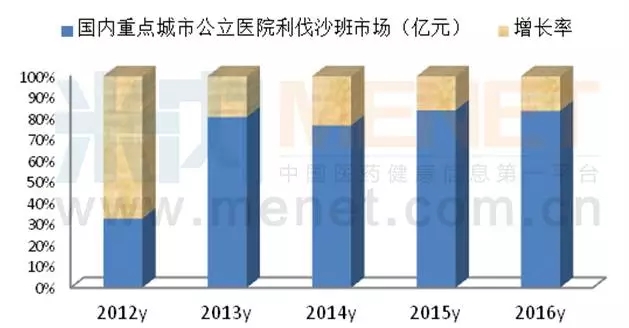
Livastaban is the first direct orally administered Xa factor inhibitor in the global market for bayer/j&j, with a global market value of $49.86 billion in 2016, up 14.20 percent year on year.The laval shaaban indications for prevention and treatment of deep vein thrombosis, is mainly used for clinical prevention after hip and knee arthroplasty patients with deep vein thrombosis (DVT) and pulmonary embolism (PE) formation, can also be used for the prevention of stroke in patients with nonvalvular atrial fibrillation and the central nervous systemic embolism, reduce the risk of recurrence of coronary syndrome and stroke.In March 2009, the CFDA approved the registration, and the product was named bairitoid.In 2016, the sales volume of livastaban, a public hospital in China's major cities, was 219 million yuan, up 42.93% from the previous year.
The growth of the domestic darbiga group increased by 149.53 percent
Dabiega is a new anticoagulant developed by boehringer ingerheim, which was approved by the FDA in October 2010.Known as the first anticoagulant oral new drug in 50 years after warfarin, it is a milestone in anticoagulant therapy and the prevention of potentially fatal thrombosis.The FDA approved the fourth indication of dabiega - prevention of deep venous thrombosis and pulmonary embolism - and thus the market growth.
In February 2013, CFDA approved the registration of dabiega group, which is named tai bi quan.In 2016, the total sales of tai bi, a public hospital in key cities in China, were 426.7 million yuan, up 149.53 percent from the previous year.Along with the wide clinical application, the domestic writing published "darby and ester group for valvular disease of clinical application of atrial fibrillation in patients with stroke prevention advice", an important step to regulate anticoagulation for oral administration, atrial fibrillation is the most serious complications of ischemic stroke, stroke prevention is an important part of the integrated management of atrial fibrillation.
Negotiate a price cut for greelo
Tigreelo is a new, selective anticoagulant drug developed by astrazeneca.In July 2011, the FDA approved a listing, called Brilinta.Tigerrillo has rapid effect, non-precursors can directly function, is not affected by individual genetic differences, and can be combined with platelet reversible binding mechanism, which can quickly restore platelet function after stopping.PLATO's study also showed that tigerrillo's efficacy was better than that of clopidogrel, which has been recommended by 10 international treatment guidelines for patients with acute coronary syndrome.
In November 2012, China CFDA approved the registration of gerriello, which is known as double Linda.As of early 2016, domestic there are 31 to submit an application for registration for Greg los, including 16 with corresponding for Greg los approved clinical, in review state, coming events cast their shadows before them.
In 2016 key cities for the domestic public hospitals for Greg sales amount is 83.93 million yuan, year-on-year growth of 59.02% a year, at present for Greg is still a exclusive varieties of astrazeneca, the domestic market reached 424 million yuan.In 2017 countries people club department released for Greg talks into medicare catalog, pricing negotiation hospital bid the lowest price by 22.78%, compared to the same for Greg lowe (90 mg) from the original 10.94 yuan/piece, fell to 8.45 yuan/piece, and became a double surplus.
|
