| Micro-channel |
 |
|
|

|
|
|
| |
| From 51 batches of Chinese medicine, imported medicine, children's medicine priority review characteristics |
| |
| Author:中國銘鉉 企劃部 Release Time:2017-8-25 11:59:31 Number Browse:790 |
| |
Medical network - August 25 from 2016 CFDA priority review so far, the examination and approval system salty V3.2 found a total of 55 production approval of data related to priority review policy of examination and approval, which ultimately didn't into the list of priority review and approval of product approval number 4, into the list of priority review and approval of product approval number 51.Four of the 51 product approvals were approved in 2016 and 47 were approved in 2017, so 2017 is the year of priority review policy.
As of August 14, 2017, there are 316 accepted number of priority reviews, 172 of which are domestic acceptance Numbers, 144 of which are import acceptance Numbers, and domestic accounting for more than half.However, of the 47 new approved approvals in 2017, only seven were domestic batches, while the remaining 40 were import approvals, accounting for 85 per cent.
In terms of approval, the average time for approval of the approved approval of domestic approval is 40 months, and the mean of the imported products is 21 months.
Domestic priority review and imported priority review, by contrast, the CFDA "imported drug registration management related matters about adjusting decision (draft)" opinion notice after the release of imported drugs approved faster, priority review products imported drugs approved by the higher success rate.
Domestic medicine: the first copy of the drug has the largest number of products
Of the 10 approved approvals, 8 of the 10 approvals were the first imitative drug (see table 1).Two exceptions: one is the simultaneous submission of us ANDA application, and it has been inspected by the FDA for the injection of azithromycin in the injection of hainan pui pharmaceutical co., LTD.The other is the anhui baker biopharmaceutical pharmaceutical co., LTD., which was approved as an anti-aids drug.
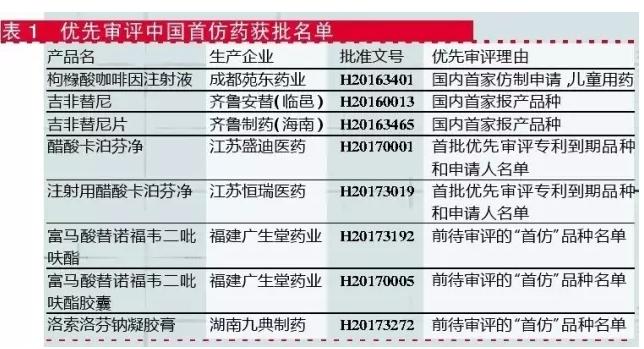
In addition, another priority review anti-hiv import drugs is noteworthy, glaxosmithkline (China) investment co., LTD., for more of the crunching of cornflakes barak, July 26, run by the state food and drug administration approved to enter the Chinese market, but as of August 14, 2017, temporarily not check the approval.
Imported medicine: most of the approved reasons are "more therapeutic advantage"
For imported drugs, the reasons for prioritizing the review are mostly "more therapeutic advantages".As shown in table 2, the author every time I see the CDE to import new priority review reason is "treatment has obvious advantages compared with the existing treatment" such comments, there is always a kind of innovative have been imported drugs "contracted" feeling.
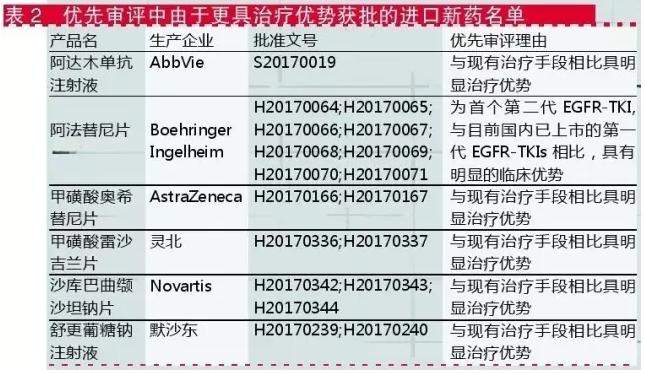
Imports from therapeutic areas, priority review of new drug approval or with targeted anti-tumor medicine is given priority to, such as the method for sheet, listed in the priority review approved mesylate shortest time for Mr Bush for his piece, treatment BRAFV600 mutation positive unresectable or metastatic melanoma d murphy's piece, treatment of primary myelofibrosis (PMF) phosphate reed can for piece, the treatment of advanced metastatic colorectal cancer (CRC) rui GeFei slices.These "new global" drugs are listed domestically and internationally, benefiting domestic patients.
Second therapy for the treatment of hepatitis c patient poison, bristol-myers squibb (China) investment co., LTD. O ShuRuiWei soft capsule and dara he wei hydrochloride in 2017 approved, combination therapy for adults with chronic hepatitis c.CFDA has also proposed the requirements for post-marketing monitoring and evaluation of the Dacca and asurrivir soft capsules.It can be seen that although the new drug is accelerated in the approval of the listed review, the relevant clinical trials should be complemented.
Children's medicine: orphan drug to be listed on the market without clinical trial, post-marketing
A total of 3 products were approved for the children's medicine, with McGonagall capsules and citrate injection as an orphan drug.

McGonagall capsule is a glucose ceramide synthase inhibitor.The product by the European drug administration in 2006 identified as orphan drug treatment of type C nyman peak disease, 2009 C nyman peak for the treatment of adult and adolescent patients of progressive neurological symptoms, then the goods have been in Australia, Canada, Switzerland, Japan and so on more than 30 countries are allowed to used in the treatment of type C NPC peak disease.Currently, the product is the only drug approved for the treatment of C - type nimanpiac disease.
In our country, in 2013, CDE's review overview column noted that "in the case of safety effectiveness, risk can be controlled, we agree that this product should be listed in conditional approval in China".Which approval conditions for post-marketing clinical trials should be carried out in domestic standard, the number of cases, 15 cases of clinical medication for at least 1 year, in this article for Chinese patients to evaluate effectiveness, safety, and dosage, understand symptom improvement situation severity and incidence of adverse reactions, adverse reaction and disposal measures, such as ".Until 2016, however, the McGonagall capsules were not approved.The original research manufacturer Actelion Registration Ltd., in 2011, declared the clinical application, declared the production in 2015, and officially approved the import in November 2016 after entering the first batch of self-check verification list.
The caffeine injection of citrate was approved in France on 31 December 1997 to treat the apnea for preterm births.In September 1998, we were eligible for an American orphan drug, and on September 21, 1999, we obtained an FDA listing permit.Currently in Europe and the United States, citric acid preparation has become the first choice for the treatment of premature infants.ChiesiFarmaceuticiSpA, which is listed in China, has not been listed in the United States.CDE, at the time, was required to carry out the open clinical trial for the treatment of premature infant respiratory suspension with the limited approval of its listing. The number of cases was not less than 200 cases.In 2016, it was approved to be the first of its kind in China.
In addition, once to children's medicine into the fitting into the list of priority review but eventually did not enter priority review list of poinsettia clindamycin hydrochloride palmitate particles and clindamycin hydrochloride palmitate dispersible tablets, and in 2017 supplementary specification.
Summary < < <
Domestic manufacturers are given priority review and approval of the first imitative drug.The "new global" new drug is a priority for importers, and the main area of impact is the market for oncology drugs.
The average approval rate for imported new drugs is faster than the domestic priority review.By table 4 shows, multicenter clinical breaks the listed listed the number of clinical and accelerate the speed, individual new drug application case number is less, after approved by CDE will ask for an increase in post-marketing clinical monitoring.

From clinical data verification to the accelerated listing of multi-center evidence recognition, it can be seen that the clinical evidence is more and more important in the process of drug development and registration.Clinical data, the drug research and development enterprise core competitiveness, the domestic research and development enterprise not only satisfied with basic management requirements of authenticity, but also from the perspective of management science and mining listed before and after the clinical data.
|
| |
Previous article:The "two-ticket system" in the three provinces has been recognized as a major reshuffle of drug distribution enterprises
Next article:The top 100 pharmaceutical industries: shandong, Beijing, zhejiang and jiangsu
|
| |
|
|
