| Micro-channel |
 |
|
|

|
|
|
| |
| Growth of over 20%! The four varieties lead the antibody drug to sprint billions of market |
| |
| Author:中國銘鉉 企劃部 Release Time:2017-9-8 15:11:23 Number Browse:1474 |
| |
Medical network - on September 8, in new drug pricing negotiations, thirty-six negotiations drugs into the 2017 edition of the national basic medical insurance, inductrial injury insurance and maternity insurance drug catalogue b class scope, the average reduction of 44%, up to 70%. Among the most notable drugs were anti-tumor monoclonal antibody drugs, including trastuzumab, bevacizumab, nituzumab, rituximab, and the average reduction of 4 drugs by 54.74%. After the antibody drug enters the national health care catalogue, it will directly promote the expansion of China's antibody market.
The big four market is 44
According to the latest authoritative data, China's biological products market reached 135 billion yuan in 2016, accounting for 9.2 percent of the total drug market, and an increase of 11.5 percent compared with 2015. The amount of drugs used in the first 100 bioengineering drugs in public hospitals in China has reached 13611 million yuan, up 13.78 percent year on year. In the past two years, the proportion of bioengineered drugs has been increasing year by year.
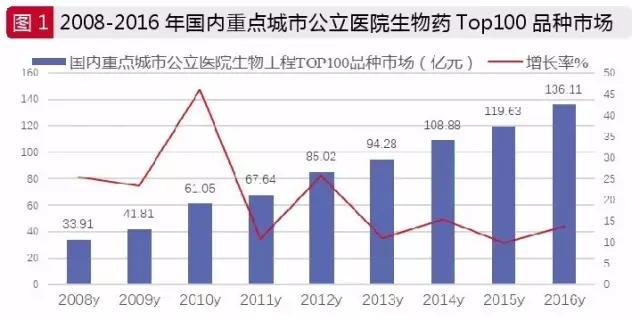
In the biological macromolecular drugs in the market, including the anti-tumor antibodies, immune stimulation, ophthalmic preparations, fibrinolytic enzymes biological biological engineering, clotting factors, growth for the subclass, biological reorganization of the hormone insulin, biology, biological vaccines, immunosuppression and biological immune globulin 12 classes, is has the development potential of varieties in clinical treatment.
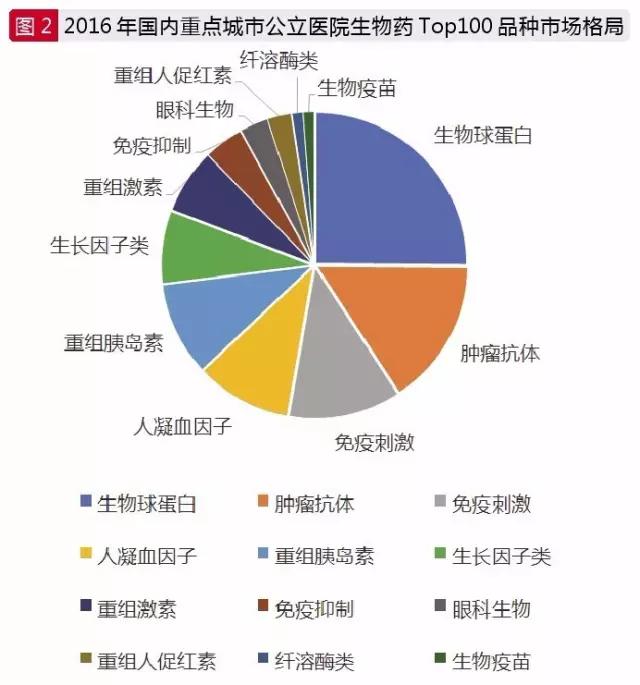
According to Thomson Reuters data, the world has now been approved and entered the Ⅲ antibody drugs a total of 243 clinical research, has won the American FDA approved varieties have 121. CFDA data show that there are 1653 copies of the approved biomolecular drug "S", and more than 95 percent of them are in the market.
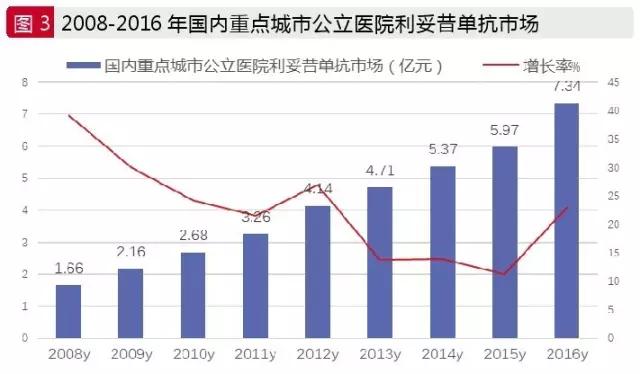
At present, the four anti-tumor antitumor drugs that are closely watched are trastuzumab, bevacizumab, nituzumab and rituximab. According to the CFDA institute of medicine economic south punctuation HDM system data information company, in 2016 key cities for the domestic public hospital four antibody drug amount is 1.846 billion yuan, year-on-year growth of 21.38% a year, four varieties overall size of the market has reached 4.4 billion yuan, account for about half of the domestic market of antibody class.
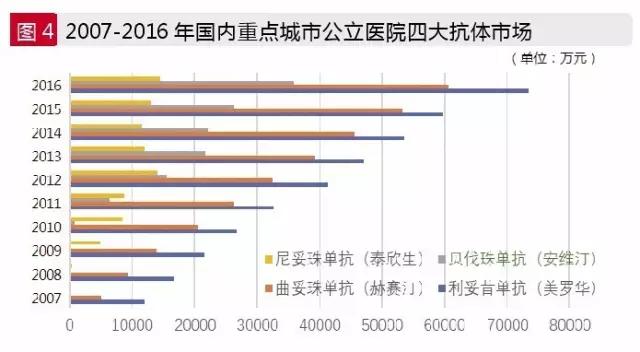
Rituximab: antibody drug leader product
Rituximab is a mainstay of roche holding, which is called Rituxan/Mabthera. In November 1997, the U.S. food and drug administration approved the listing. In 2000, China approved the listing of lituximab, which was named "meiluo hua". Rituximab is a gold standard for non-hodgkin's lymphoma and is approved as a first-line drug for advanced colon cancer. With the development of clinical medicine, the theory of anti-tumor therapy for the antitumor therapy of the same genotype, different location and different tissues has been advanced comprehensively. At present, rituximab has been approved for the new indications of immune diseases such as rheumatoid arthritis, promoting the rapid development of monoclonal drug market.
According to the data of HDM system, the amount of rituximab in public hospitals in the major cities of China was 734 million yuan in 2016, an increase of 22.94% over the previous year. The total market of lituximab in China has reached 16.67 billion yuan, showing sustained growth.
The results of the new round of drug price negotiations showed that the 50ml of us $500mg was reduced from 16041 yuan to 8289.87 yuan per unit, with a decrease of 48.32%. Another specification 10ml: 100mg injection was reduced from 3,416 yuan to 2418 yuan per unit, down by 29.22 percent. Clinical for recurrence or resistance of the central type of lymphoma, follicular CD20 positive Ⅲ - phase Ⅳ follicular non-hodgkin's lymphoma, CD20 positive diffuse large B cell non-hodgkin's lymphoma.
With the expiration of lituximab, the research and development of biosimilars has been carried out in China. According to recent data, the CFDA has several domestic enterprises get clinical approval, it will be fine as zhengda pharmaceutical group of rituxan (CXSL1500056 Sue), westlands of genetic engineering restructuring antilymphocyte tumor (CD20) single resistance (rituxan) injection (CXSL1400096 yu), sea cisco biological drugs "HSK - Ⅲ - 001 injection" l10582 (2016). When the domestic rituximab has been listed, it will expand its use and is expected to replace parts of the us.
Trastuzumab: breast cancer treatment continues to grow
By bead sheet resistance is roche's another backbone varieties, FDA approval in 1998, is the first approved for the treatment of metastatic breast cancer and breast cancer early human epidermal growth factor receptor 2 (HER2) monoclonal antibodies, are widely used in each phase of positive HER2 breast cancer treatment, commodity called Herceptin. Roche's sales of trastuzumab in 2016 amounted to 7.613 billion Swiss francs, up 4.19 percent year on year.
In 2003, roche's trastuzumab injection entered the Chinese market, with the name herceptin. In 2016, the total amount of anti-drug use of trastuzumab in public hospitals in China was 607 million yuan, an increase of 14.2% from the previous year. The total market of trastuzumab in China has reached 1.19 billion yuan, and the market for anti-breast cancer treatment has continued to grow.
A new round of drug price negotiations, according to the results of herceptin 440 mg injection by negotiation before bidding price 21613 yuan/dropped to 21613 yuan /, fell to 64.80%, is the highest anti-tumor drugs in the drop.
With the expiration of trastuzumab, generic drug research and development has been carried out in China. According to recent data, the CFDA in 2016 there were two companies received clinical approval, respectively is zhengda pharmaceutical group, the weather is fine for injection by bead sheet resistance (CXSL1400013 Sue), westlands genetic engineering the restructuring of the resistance to epidermal growth factor receptor 2 (HER2) single resistance (by bead sheet resistance) injection drug use (CXSL1400077).
Bevacizumab: new indications for increased sales
Bevacizumab is roche's blockbuster, and the world's first antitumor angiogenesis drug. In February 2004, the United States FDA approved the treatment of metastatic colorectal cancer. The product was called Avastin. Currently, the drug's indications include colorectal cancer, non-small cell lung cancer, platinum-resistant ovarian cancer, cervical cancer and kidney cancer.
Bevacizumab officially entered the Chinese market in May 2010, and the product was named anvidine. The first indication was advanced metastatic colorectal cancer, combined with 5-fluorouracil or ealiticonine for late metastatic colorectal cancer. In August 2015, the CFDA approved a first-line treatment for advanced, metastatic or recurrent non-squamous cell lung cancer. Bevacizumab combined with carboplatin taxol chemotherapy can bring obvious benefits to patients with advanced lung cancer and reduce the risk of death.
In 2016, the amount of bevacizumab in public hospitals in China's key cities was 360 million yuan, up 37.43% from the previous year. The overall market of bevacizumab in China has reached 1.96 billion yuan. After an increase in fitness, avantine's 2016 sales were up 63% from 2014.
According to the results of the drug price negotiation, anvistatin 4ml: 100mg injection was reduced from 5176 yuan/branch to 1998 / branch, with a decrease of 61.40%, which was the second largest reduction in anti-tumor drug.
Nitozumab: the first anti-cancer antibody in China
Nitozumab is the first functional antibody drug used to treat malignant tumors in China. In 2008, the launch of the 100 - tai creature, named tai xin sheng, broke the foreign monopoly for the first time.
Nitozumab is the world's first monoclonal antibody to target epidermal growth factor receptor (EGFR). Tai xin combined radiotherapy and chemotherapy treatment of nasopharyngeal carcinoma, head and neck cancer, glioma, colorectal cancer, pancreatic cancer, esophageal cancer, liver cancer, non-small cell lung cancer and other solid tumor, is a high selectivity, good safety antibody drugs, to specific targeting therapy for solid tumors cells.
In 2016, the amount of the drug used in the public hospital of the key urban public hospital in China was 144.5 million yuan, up 11.96% from the previous year. Since the listing, the drug market of nituzumab has exceeded 2 billion yuan in the domestic public hospital.
A new round of drug price negotiations, according to the results of tai xin born 10 ml: 50 mg injection from RMB 2378 / dropped to 1700 yuan/branch, fell to 28.50%, limit and radiotherapy combined epidermal growth factor receptor (EGFR) expression of positive Ⅲ/Ⅳ stage of nasopharyngeal carcinoma. It is expected to increase market share after cutting prices and entering medical insurance. If it can be promoted to class a, the market share will be greatly increased, which will prompt more enterprises to invest in new drug research and development.
|
| |
Previous article:增速超20%!四大品種引領抗體藥沖刺百億市場
Next article:9 departments to jointly fight food, health food fraud and false propaganda!
|
| |
|
|
