Medical network - on December 28, as of December 21, 2016, the FDA has approved a 21 new molecular entities drugs (small molecule drugs and biological products), approved quantity is 45 significantly lower than that of last year.
"The reality is very cruel." Corning jerry chairman Xu Ting to medicine economic newspaper reporter pointed out that "global pharmaceutical companies are gazing into the U.S. market, but the FDA approval of new drugs each year is hammering a wooden bridge, more than 40, less then 20. The approved varieties, market revenues and only a small part of the sales of more than $1 billion less left."
"If we can't keep a clear head, the" hot "today becomes tomorrow's 'cold'." In the research and development of simcere's chief scientific officer and President Dr MouHua view, how to understanding of the risks involved in new drug research and development way, and then puts forward an effective risk control plan, is all drug companies must face the issue. "Who can handle well, who are likely to be a winner!"
【 origin 】 in failed more than half of all new drugs (Ⅱ clinical stage, enterprises should do?
- a logic: target innovation step by step
In recent years as a large number of returnees talent courageously into new business, the level of domestic drug discovery (discovery) began to gradually in line with international standards. But one cannot ignore the fact that most of this group of scientists from research and development, new drug projects tend to start from a new targets or new technology, "this product is really is a clinical need? From the new drug project to clinical and a series of steps, including new drug (IND) declare, different clinical stages of testing scheme design, the expert is lack." Yu bearing capital partners Zhu Zhongyuan said publicly.
Nature Biotechnology, involving 835 drug firms, 4451 new drugs, 7372 kinds of indications of data statistics, the results showed that a new drug from preclinical to clinical and listed the probability is only 10.4%, which involves proof-of-concept (PoC) Ⅱ phase of clinical trial the highest failure rate. According to the data of drug development research center at tufts university, new drugs from clinical stage Ⅱ to Ⅲ stage conversion rate at about 40%, more than half of the new drug failure in Ⅱ phase of clinical trial.
"This is a key way for the innovation in the process of drug development, the industry must pay some cost can be really grow up." Now a large number of new drugs into the middle distance clinical trials and for a period of time, but some experts relentlessly with the flourishing new drug research and development of domestic poured cold water.
The reporter understands in the interview, there is a very important in the process of clinical trials of nodes -- proof-of-concept (PoC). Once the drug targets by PoC, basically can prove biology path, research and development of systemic risk is reduced greatly.
"To achieve PoC will tell from the world is difficult, at present Chinese enterprises still lack of experience from the early discovery to the PoC, especially the relatively high degree of innovative medicines." MouHua served as hutchison whampoa's chief medical officer, he admits, hutchison whampoa, as one of the earliest domestic enterprises to innovative drug research and development, also from the relatively mature EGFR inhibitors and VEGFR inhibitors increase the target level of innovation, step by step growth to high risk and difficult c - Met inhibitors.
A number of the personage inside course of study points out that the domestic innovative drug research and development has just started, is at the early stage of innovation of enterprise, to accumulate through long-term me too, fast - follow - on and after I could experience gradually transition to the new innovation, "innovation medicine research and development is not achieved overnight."
【 origin 】 the domestic development first - in - class "new world" is not yet ripe, layout of domestic medicine companies have good?
- logic: rich product line level
MouHua believes that the current domestic company independently developed the timing of the first - in - class drug is not yet mature, even top innovative enterprises, all products are independent of the "new" is not reality, but that doesn't mean you can't touch the world "new", instead of prompt enterprise to carry on the reasonable planning, product line to pay attention to collocation.
At present the most complete layout of the traditional medicine enterprise in the field of innovative medicine hengrui pharmaceutical, for example, in the development of new drugs hengrui took a risk/reward more balanced "fast track" strategy, layout of the reentry after PoC success, depend on quantity and speed of subsequent spell project, on the other hand, the company also exports to a large number of generic drugs and drug products business as a buffer, and "generics medicine + innovation" business portfolio also meet the clinical needs of the diversification.
An innovative drug firms. Xu Ting told reporters that the current corning jerry on the one hand, following the current tumor immune targets for PD - L1, CTLA4, etc, on the other hand also establish cooperation relationship with professor college scientific research institutes, some layout to the forefront of innovation targets. In addition, the company also similar to transfer nearly 30 biological medicine formed cooperative development network, which laid a foundation for potential new drugs in the future cooperation
"Few opportunities for single drug resistance to avoid the homogeneity competition, protein drugs may have an advantage in the short term." Xu Ting said, such as long-term estrogen, follicle "valuable but no business concern" the differentiation of drugs is also an important part of its product line.
As more and more drug firms in new drugs, the status of innovative drugs in the enterprise product line more and more important. How to circumvent the risk of research and development, the limited funds value maximization, become both traditional drug firms and innovative drug firms face common challenges. MouHua is instructive and puts forward some ideas of the layout of the or, for a relatively mature targets, using their own independent market development in China, for the high degree of innovation of the "new" drugs are actively seeking international strategic cooperation.
【 origin 】 cooperative r&d project success rate is higher, so blindly "outsourcing"? And how does its own research and development ability?
- three logic: internal and external research and development ability
"From simcere strategic point of view, we don't seek to buy imported first - in - class varieties. The risks and benefits is coexist, before the experience and strength did not reach, we prefer to cede more benefit to jointly develop, share the risks." MouHua said.
"Mountain, can offend jade." This experience with hutchison team of scientists from MouHua three global multinational companies to establish strategic cooperation experience. - Met with one of the c inhibitors, for example, in the realized it was the highest level of innovation in the product line, the greatest risk of new drugs, after he and his colleagues decisively put forward the suggestion of cooperation: "working with big drug firms, not only can solve the problem of 'one' money, at the same time, the experience of using the partner can reduce the drug fast forward."
MouHua then puts forward a set of detailed Chinese and international development path, it was also based on this, attract the cooperation of astrazeneca, the drug is currently in Ⅱ phase of clinical trials. "A clear mind, a detailed planning, a set of complete, also can make a decent new drugs of Chinese enterprises."
According to McKinsey analysts from 1996 to 2014, more than 9200 kinds of candidate drugs in clinical success rate statistics and registration phase, cooperative r&d project has always maintained a higher success rate. Data show that in 1998, cooperative research and development of drugs from Ⅰ period clinical to approved the total pass rate was 32.2%, compared to 11.5% of cooperative research and development of drugs has obvious advantages. By 2010, the probability, down 12% and 4.3% respectively, but the cooperation development mode still about 8% higher. Between 2009 and 2014, cooperative research and development in the late clinical success rate of lead were improved significantly.
"When you are not sure whether enterprise early technology to ensure product success, cooperation with big drug firms is a way of development diversification." As biological coupling field type platform technology company, Ambrx with bristol-myers squibb, Merck, lilly, multinational drug companies and a number of cooperation on a series of projects. , according to chief scientist Dr Ford Ambrx Ambrx early in development when the proportion of independent research and development and the cooperation about 3:7, after mature technology to a certain extent, is the transition to "give priority to in order to develop its own product lines and cooperative development as the auxiliary pole" on the direction of the proportion has been turned into 7:3.
Currently Ambrx through technical cooperation projects and achieved more than $250 million, and in May 2015 by fosun medicine, magnolia bark joint investment, everbright, wuxi bid.
Created the value of new drugs are no longer confined to the downstream industry chain. In fact, in addition to the cooperative development, domestic patterns of drug research in recent years, more and more flexible, traditional dragon "closed-door medicine making" process is broken gradually, instead is a delegate with "VC + IP + CRO" research and development of a variety of business model of the relay.
New business model
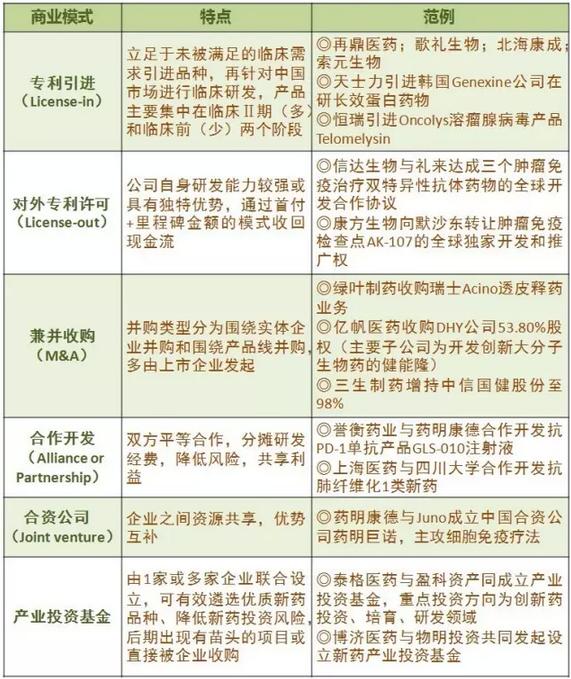
But in Xu Ting view, a business to achieve long-term development, must develop their own research and development capabilities. "I think the enterprise through their own efforts to form the core competitiveness is very important, the core competitiveness, technology may be your team, also may be many years research and development and accumulated experience of cooperation. In theory, should be dominated by innovation, small business information more developed now, you can see the other people all can see, if blindly rely on outsourcing services, you may want to know more where is the advantage."
【 origin 】 the sales revenue is proportional to the r&d, but domestic innovation medicine profit is limited, how to get high enough returns to support r&d?
Four - logic: targeting the international market
A engaged in new drug registration in the industry have told reporters, "I often suggest innovation medicine enterprises, if the rich have power, can consider to go to the United States, Australia, or synchronous declare China Hong Kong, China Taiwan, these places for examination and approval of speed is much faster than mainland, Australia and even the corresponding research subsidies, make full use of the conceptual validation/macau for examination and approval of the poor do help to accelerate the early clinical research progress. More importantly, these countries and the area of medical insurance payment system more perfect, innovative drug post-marketing profit space security."
A website has global sales of the top 23 large pharmaceutical companies from 2011 to 2015 drugs research and development (R&D) investment and sales income has carried on the statistics, the results showed that the compound growth rate of R&D and sales compound growth correlation coefficient is 0.9. Sales revenue, in other words, to a large extent determines the development of the enterprise.
According to public, according to data from 2011-2011, the annual spending on research and development of pharmaceutical enterprises in China sales than the industry average of around 3%, the highest reached 9% ~ 9%, the figure with global multinational drug companies and a large gap compared to about 20% of spending on research and development. "European and us companies dare to hit so much money on research and development because profits there, Chinese innovation medicine payment mechanism is not perfect enough, for such a big investment, enterprise can gain enough to support the return?"
Experts questioned in the interview, the input-output contradictions of market in China, become a big test for drug firms decisions. Particularly dependent on drug patent introducing enterprise, on the one hand, is a research and development is a large risk, license fee is no small expenditure; On the other hand also do clinical trials for the Chinese market to develop, the enterprise how much probability to find a good project, how to hold return?"
"Of this small company size, we do here in new drug research and development is likely to be or want to the international market to gain recognition, and is large enough to produce the value of the profit space." Xu Ting said. |
